Central venous pressure (CVP) is an important clinical indicator of progressive cardiac diseases. Venous congestion can be quantified by CVP, and its monitoring is essential to understand and follow the hemodynamic status in patients with heart failure (HF) and renal/hepatic functional alterations. Nowadays, CVP can only be measured in the hospital and not as frequently as would be necessary. The new VenCoM device allows for non-invasive and reliable measurement of venous pressure. Its ease of use makes it suitable for use not only by specialized personnel in a hospital or outpatient setting but also directly by the patient for home monitoring
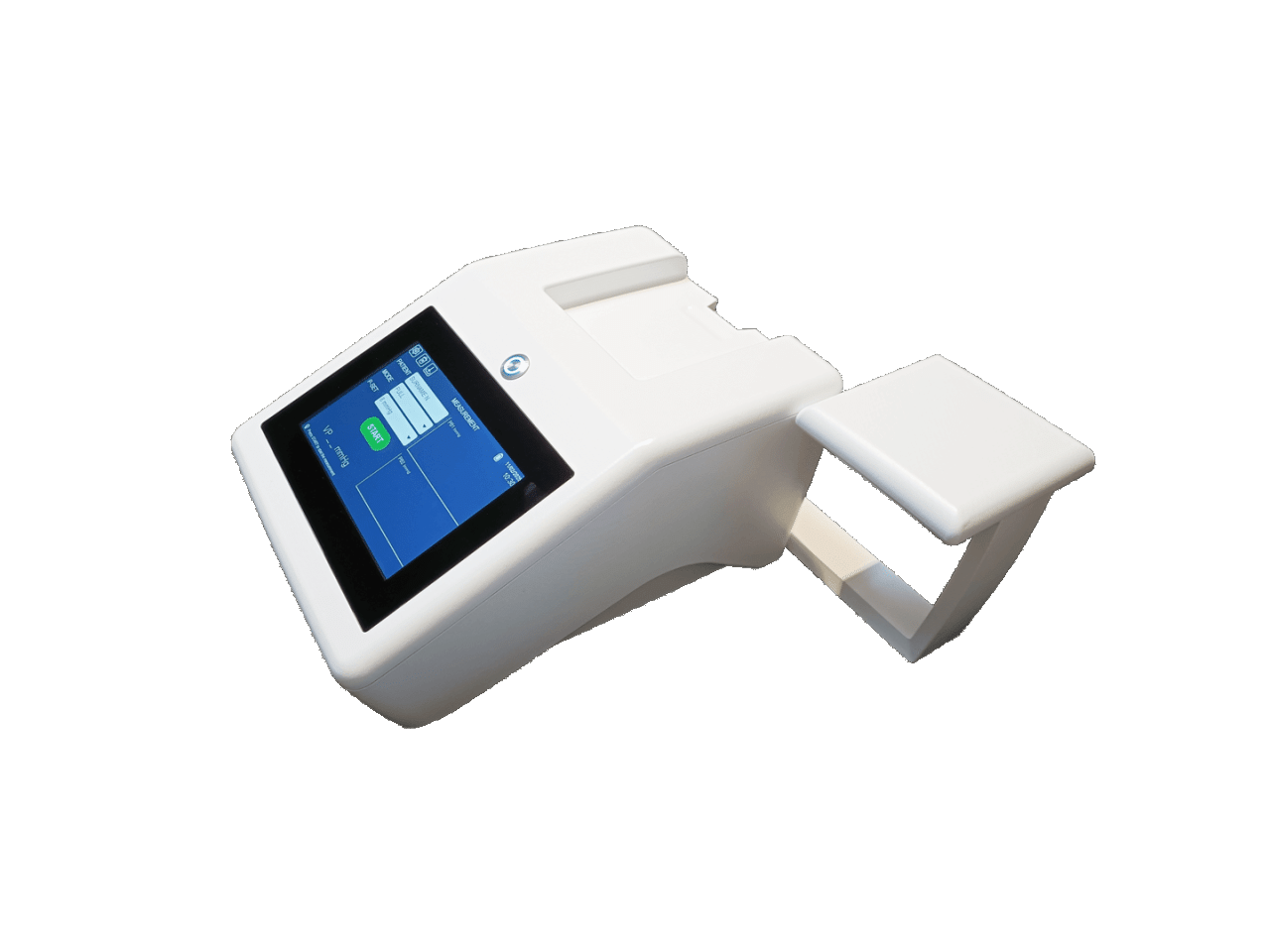 Fig.1: VenCoM Device
Fig.1: VenCoM Device
VenCoM (Venous Congestion Meter) is a non-invasive device suitable for measuring venous pressure in hospitals, outpatient settings, or at home.
It is a plethysmographic device similar to a standard sphygmomanometer for measuring blood pressure, but equipped with two pneumatic cuffs: one positioned on the upper arm to occlude venous flow when the applied pressure exceeds the existing venous pressure, and the other positioned on the forearm to detect volume changes induced by occlusion as a result of rapid vascular distension in the forearm. Based on the interactions of these pressures applied to the arm and the related variations detected in the forearm, and thanks to an innovative algorithm, the device determines the estimated CVP value within a few minutes.
Regarding the VenCoM device, Teoresi MedTech has overseen the entire development cycle of the device in compliance with European medical regulations, from the specification of requirements (system, hardware, and software) to the production and validation of the device. In particular, the HW components were selected and the electronic boards were designed in accordance with the required functionalities. Regarding the SW design, all architectural and detailed design activities of the software were carried out to implement the required functionalities
The main challenges in developing a new solution for venous hemodynamic monitoring included:
- providing a non-invasive, easy-to-use, and portable monitoring system;
- delivering a measurement that is reliable and accurate compared to the current invasive gold standard;
- making this monitoring solution accessible to more patients and healthcare providers
Nowadays, central venous pressure (CVP) can only be measured in hospitals and not frequently. Cardiac catheterization is the clinical gold standard for CVP assessment. However, it is risky, uncomfortable for the patient, and limited to hospital practice. Non-invasive alternatives suffer from poor reliability, are operator-dependent, and are not suitable for home use. In contrast, VenCoM is a non-invasive device suitable for measuring venous pressure in hospitals, outpatient settings, or at home
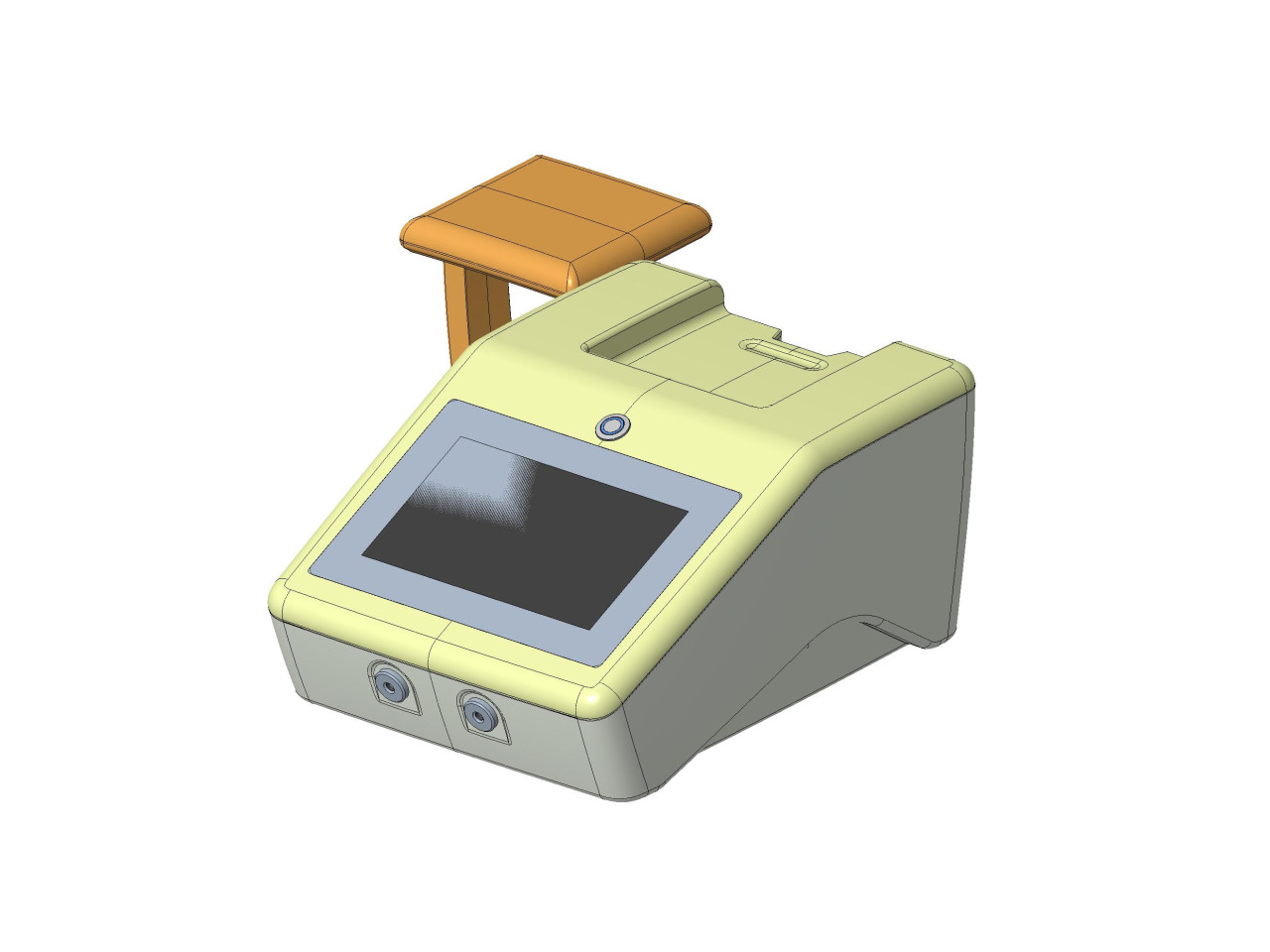 Fig.2: 3D rendering of VenCoM device
Fig.2: 3D rendering of VenCoM device
The VenCoM device, being a non-invasive, easy-to-use, and portable monitoring system, can be used for measuring venous pressure in hospitals, outpatient settings, or at home.
Specifically, it is necessary to position the two pneumatic cuffs, one on the upper arm and the other on the forearm. Through the user interface, it is possible for all users to operate the machine to perform the measurement and obtain the estimated CVP value within a few minutes.
The VenCoM device provides crucial information about patient's health status and enables early detection of potential venous congestion crises, potentially predicting acute HF events several days in advance—events that typically require hospitalization. By preventing acute decompensation events and reducing hospital admissions, patients experience improved quality of life and increased life expectancy.
Key benefits of the VenCoM device include:
- It is non-invasive and ensures patient safety and comfort
- It is easy to use, as its intuitive design is suitable for both healthcare providers and patients
- It is highly reliable, providing accurate measurements
- It is versatile, effective for both hospital and home monitoring
- It is inclusive, as it is suitable even for patients with arrhythmias
- It is easily integrable with standard blood pressure monitors
TRE ESSE Progettazione Biomedica s.r.l.
A patented technology for non-invasive measurement of venous pressure
Teoresi MedTech is involved in the development of the hardware/software components of the VenCoM device based on project requirements defined by TRE ESSE and in compliance with the current European Medical Device Regulation
 Fig.3: Application of VenCoM device (including pneumatic cuffs)
Fig.3: Application of VenCoM device (including pneumatic cuffs)