Extracorporeal organ support therapies are increasingly spreading for the treatment of critically ill patients. Extracorporeal membrane oxygenation (ECMO) and carbon dioxide removal (ECCO2R) replicate the gas exchange functions of native lungs, whereas renal replacement therapies (RRT) are used for solutes or fluid removal. The future of critical illness is moving towards the development of multi-organ support devices that offer multiple treatment lines on a single hardware platform. MediCon has designed and validated a device for multi-organ support, developing an auxiliary line for renal support (hemofiltration) to be integrated on-demand to a ECCO2R main line, with the aim of managing fluid overload complications by ultrafiltration. Furthermore, an ultrafiltration regulation system has been developed using a powered and software-modulated pinch-valve on the effluent line of the hemofilter, proposed as an alternative to the state-of-the art solution with peristaltic pump.
The hemofiltration line (green) is connected in parallel with the main ECCO2R line, by means of a shunt that branches off from blood sampling line (blue). MediCon has developed the whole platform for combined ECCO2R – Hemofiltration treatment, from both hardware and software point of view. A syringe pump was developed for the automatic infusion of anticoagulant solution, as well as a blood leakage detector on the ultrafiltration line and two motorized clamps for the automatic closure of circuit line sections (ultrafiltration clamp and return line safety clamp). Three peristaltic pumps are also used: the main blood pump, one for perfusion of hemofiltration section, and one for replacement fluid infusion (optional). MediCon has developed A stepper motor control board has been developed by MediCon, for driving pumps and clamps. Circuit pressures are measured through a non-invasive system composed of a surface sensor and a plastic dome, and the dome holding system was developed by MediCon. Air bubble sensors are also used, and two load cells for weight monitoring of fluid collection bags. On the hemofiltration line an innovative system has been developed for net ultrafiltration rate regulation, for the removal of excess plasma water from whole blood. The system has been validated with or without contemporary infusion of replacement fluid and with both low-flux and high-flux hemofilters.
The ultrafiltration system consists of a pinch-valve (clamp) equipped with an actuation system, which drives an intermittent opening. The clamp opening is modulated via software with a closed-loop control system, based on the real-time measurement of the ultrafiltrate volume collected and any replacement fluid infused. The ultrafiltration process, during the clamp opening phase, is passively guided by the pressure drop on the effluent line (water column), thus avoiding the use of a dedicated peristaltic pump. The hydrostatic pressure of the blood flow within blood compartment of hemofilter (thrust force) and the attractive force exerted by the water column on the effluent line (suction force) contribute to the transmembrane pressure gradient necessary to activate the ultrafiltration process without active forces, reducing strain on the filter membranes especially in the presence of hemoconcentration. With a reduced and constant pressure on the ultrafiltration line a dedicated sensor for monitoring the transmembrane pressure is avoided, with consequent simplification of the device hardware and disposable circuit compared to standard peristaltic pump solution.
The ultrafiltration system technology can be used both to design an auxiliary hemofiltration line to be integrated with other treatment lines with the aim of developing a multi-organ support device, and to design a stand-alone device for renal replacement therapy. The hemofiltration line can also be adapted for blood purification treatments such as hemoperfusion and plasmapheresis. Furthermore, the ultrafiltration rate regulation system could be further developed for diffusion-type renal replacement therapies, with the use of counter-current dialysate solution in the hemofilter effluent line.
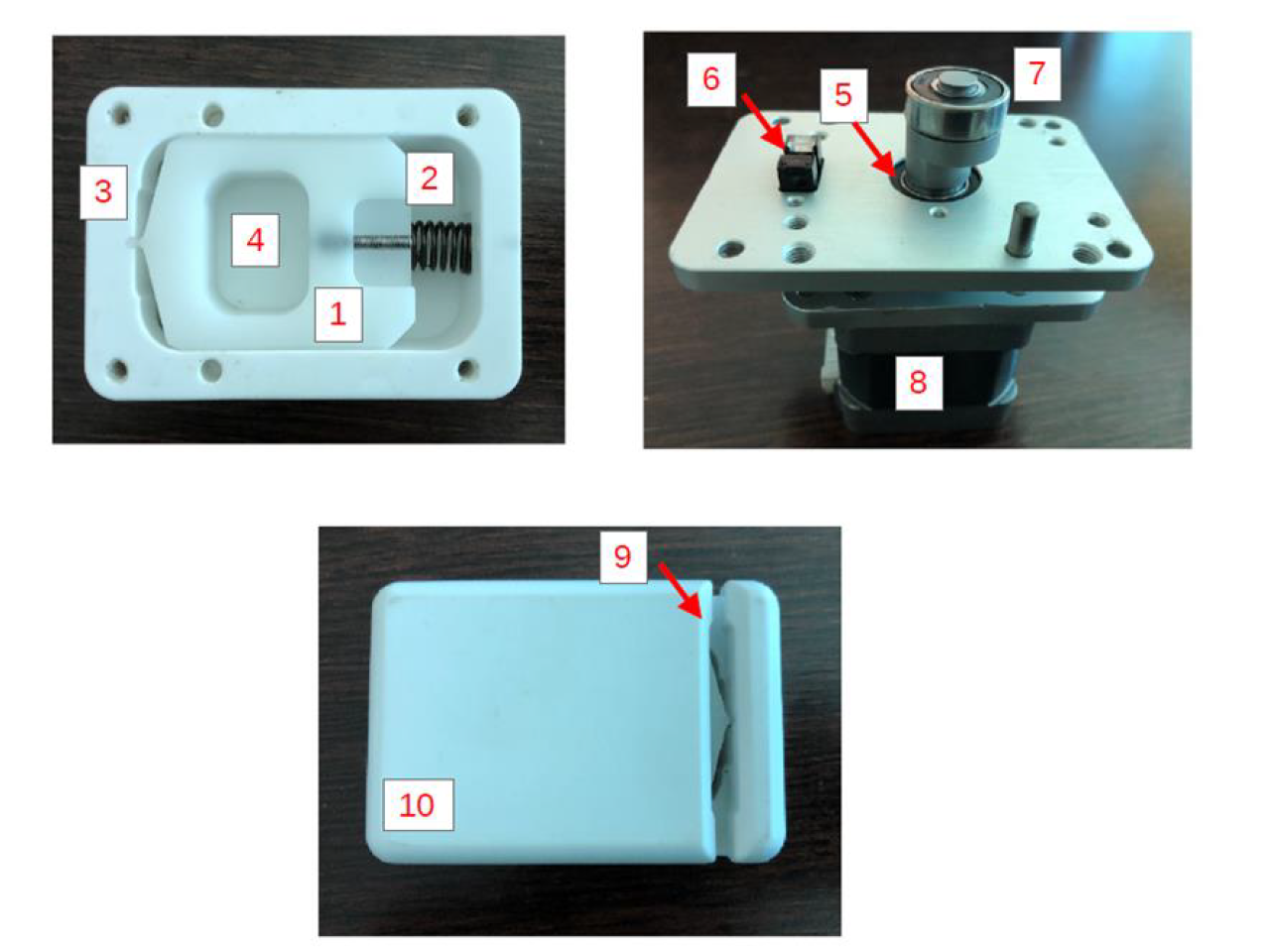 Mechanical parts of the motorized clamp for ultrafiltration rate regulation developed by MediCon
Mechanical parts of the motorized clamp for ultrafiltration rate regulation developed by MediCon
The ultrafiltration rate regulation technology has been applied to a device for combined ECCO2R-hemofiltration treatment, in which the contemporary infusion of replacement fluid allows to manage the fluid balance with the aim to achieve overall volume control. It has also been used in a device for isolated ultrafiltration (slow continuous ultrafiltration, SCUF), i.e. in the absence of a replacement line, for the treatment of hypervolemia in congestive heart failure patients
The hemofiltration platform technology developed by MediCon has been tested and validated in the operational environment, both in stand-alone mode and in combination with the ECCO2R line for the multi-organ support device, and in both cases a Technology Readiness Level has been reached equal to TRL 9. Tests have shown that the ultrafiltration rate regulation system is reliable and accurate under different operating conditions, i.e. with different ultrafiltration setpoints, with high or low hydraulic permeability hemofilters and on different hardware platforms. The trend of the patient's weight loss (fluid removal volume) follows the set one with high correlation (R2> 96%) and the desired weight loss target is achieved in the expected time. The ultrafiltration rate follows the set point with reduced fluctuations, and the variability with respect to setpoint always remain within the parameters defined by the EN 60601-2-16 standard. Compliance with international directives and standards and risk analysis process in accordance with the ISO 14971 standard make the haemofiltration line suitable for clinical use, in compliance with general safety and performance requirements. The device is currently CE marked and used regularly in clinics and hospitals.
Università di Bologna, Eurosets Srl (Medolla)
The result of this research has made it possible to create a technological platform that MediCon offers on the market in order to create new collaborations with potential new customers.