Pulmonary administration of protein drugs or vaccines in the inhaled powder form is an alternative to administration by injection. The technology stems form the project Pulmomed (www.pulmomed.eu). Proteins at high purity level were expressed with a controlled fermentation process. Respirable microparticles containing proteins in stable form were prepared for spray drying by carefully balancing the formulation components and the drying conditions. The powders obtained are administered by inhalation using a portable device for dry powders.
 Caspule containing alfa-1 antitrypsin and the inhaler
Caspule containing alfa-1 antitrypsin and the inhaler
Proteins with therapeutic, are becoming more and more important in clinical applications. The effective administration of such drugs via a non invasive and patient friendly mode represents a significant innovation. Proteins are molecules difficult to handle, formulate and store. The innovative aspect of the technology is the integrate approach between protein expression and purification and powder formulation. This allow for the stabilization of the protein in the solid state as well as for particle engineering to maximize the lung deposition.
A technology for the production of respirable microparticles for the administration of protein drugs is of great interest for the pharmaceutical industries dealing with the development and commercialization of curative or preventive vaccines, protein with therapeutic or diagnostic actions (or both such as in the case of the so called theranostic molecules) against pathologies localized in the lung. The technology is however suitable also for producing in a easy and reproducible manner stable protein-containing powders that may be administered also via other way
 Schematic representation of the process for the protein-conteining respirable powder
Schematic representation of the process for the protein-conteining respirable powder
Innovation in the treatment of lung deseases AAT deficiency
Alfa-1 Anti Tripsin (AAT) deficiency is a hereditary condition that lead to an increased risk of chronic obstructive pulmonary disease (COPD), emphysema and chronic bronchitis. People with AAT deficiency are exposed to a significant risk of lung function degeneration, that may affect their quality of life and life expectancy. AAT protects lung tissues by inhibiting the destructive enzymatic action of elastase. Elastase physiological function is related to digestion of bacteria and other foreign particles in the lungs. When a patient with AAT deficiency inhales irritants or gets a lung infection, the neutrophil elastase released in the lungs continues to act uncontrolled, and this leads to destruction of healthy lung tissue. This process may cause emphysema, significant deterioration in lung function and eventually total destruction of lung tissue. AAT deficiency can be treated with an augmentation approach by administering exogenous AAT to increase the AAT level. The only drug product presently available for local AAT administration in the lung is a liquid formulation that is delivered by aerosolization which is a techniques with several drawbacks
Chiesi Farmaceutici SpA, Bormioli Pharma srl
The value stemming from the technology is partly related to the know-how for protein stabilization in form of powder. The technology for vaccine administration is being protected by a patent application.
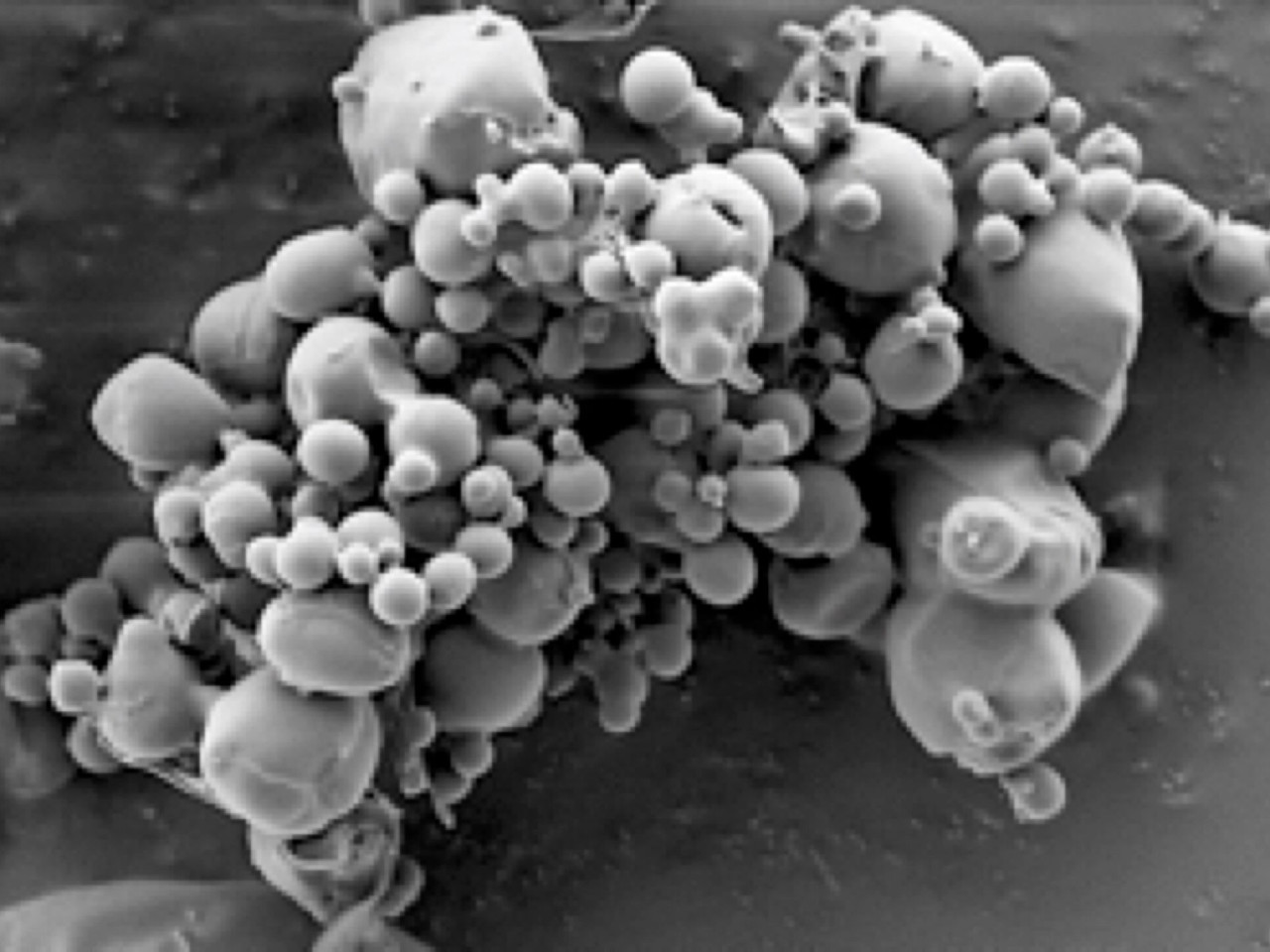 SEM pictures of AAT-containing microparticles
SEM pictures of AAT-containing microparticles