The characterization of the mutational asset and transcriptional deregulation of hematopoietic stem cells of patients with myelofibrosis, through genomic and transcriptomic analysis at the single cell level, is one of the projects of the CIDSTEM laboratory in the field of personalized medicine.
The project aims to analyze single hematopoietic stem cells from patients with myeloproliferative neoplasms, rare chronic haematological disorders that frequently evolve to acute leukemia with poor prognosis.
The characterization of the clones that support the pathogenesis of the disease, from the early stages to the evolution to acute leukemia, together with the identification of new signaling pathways involved in the disease progression, are the basis for the development of personalized therapies.
The expansion of the cohort analyzed with this method will allow to improve the current prognostic models and address to personalized therapeutic choices
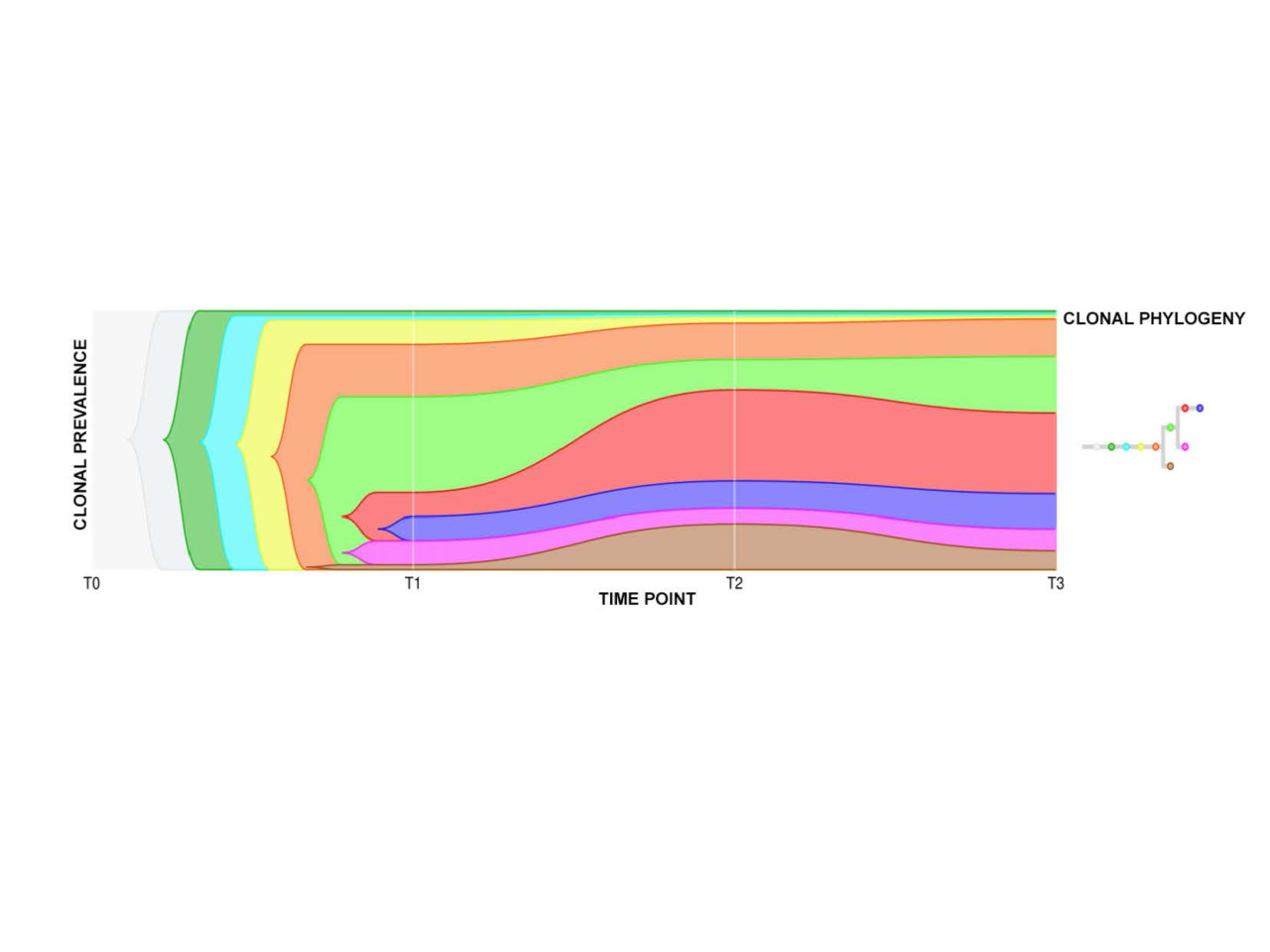 Representative diagram of the clonal architecture of a myelofibrosis patient. The different colors indicate the different mutations found in stem cells and their relative abundance.
Representative diagram of the clonal architecture of a myelofibrosis patient. The different colors indicate the different mutations found in stem cells and their relative abundance.
The specific goals of the project are:
- the optimization of a single cell genomic analysis tool that allows to characterize the clones that support the pathogenesis in early stages of the disease and its evolution to acute myeloid leukemia;
- the development of a workflow for single cell transcriptomic analysis, in order to identify new signaling pathways involved in the evolution of the neoplasm, which can be targets of new therapeutic approaches;
- the extension of this protocol to all hematological neoplasms and solid tumors, supported by multiple clones whose prevalence is modulated during disease progression.
Single cell genomic and transcriptomic analyses are the new frontier in the study of neoplasms. Recent studies have in fact shown that the mutation acquisition order influences patients’ response to treatments. This project aims to characterize the clonal complexity of myeloproliferative neoplasms. This study belongs to the rapidly evolving field of personalized medicine.
Single cell methodologies developed in the project can find application not only in the field of haematological tumors but also in solid neoplasms affecting all other organs. The study model can be applied to all neoplasms, characterized by a wide clonal heterogeneity, providing important information on their pathogenesis and evolution. Moreover the analysis will allow to correlate the occurrence of specific mutations with the prognosis of each patient, thus enabling to optimize personalized treatments.
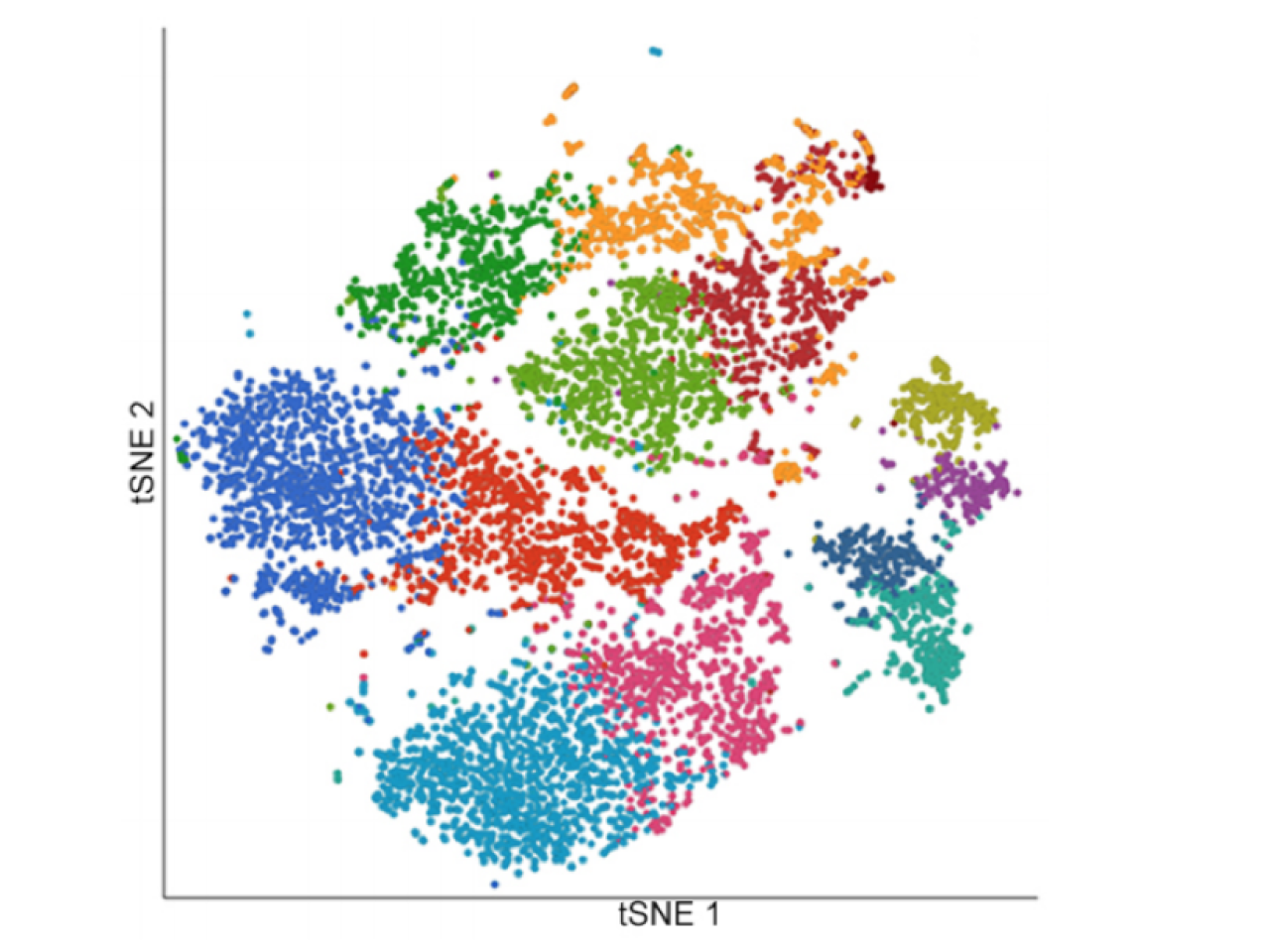 Representation of the spatial arrangement of each cell based on its transcriptional profile.
Representation of the spatial arrangement of each cell based on its transcriptional profile.
Early identification of clones that support tumor progression, implementation of prognostic criteria and identification of personalized therapies according to the clonal architecture of each patient.
CIDSTEM researchers have successfully developed a single cell genomic analysis protocol aimed at characterizing the mutational status of patients with myelofibrosis (Carretta C., Mallia S. et al, IJMS, 2020). The protocol was applied to the stem cell analysis of a patient diagnosed with myelofibrosis, followed during the progression of the disease, confirming the strength of the optimized workflow and the importance of clonal complexity study (Parenti S., Rontauroli S. Carretta C, Mallia S. et al., Precision Oncology 2021).
CD34 + hematopoietic stem cells are isolated from the patient's peripheral blood and characterized by genomic and transcriptomic analysis. This study allowed the early identification of small clones, bearing a particular mutational asset, capable of expanding and determining the malignant evolution of the neoplasm. This expansion reflects the worsening of the patient's global conditions and its non-responsiveness to therapies.
CIDSTEM, CGR UNIMORE, AOU Modena, Holostem Terapie Avanzate, Chiesi Famaceutici, JSB, Fondazione Democenter-Sipe
Carretta C, Mallia S, et al., Genomic Analysis of Hematopoietic Stem Cell at the Single-Cell Level: Optimization of Cell Fixation and Whole Genome Amplification (WGA) Protocol. Int J Mol Sci. 2020 Oct 6;21(19):E7366.
Parenti S, Rontauroli S, Carretta C, Mallia S et al., Mutated clones driving leukemic transformation are already detectable at the single cell level in CD34-positive cells in the chronic phase of Primary Myelofibrosis. Precision Oncology,2021
 GMP operator at CIDSTEM Labs
GMP operator at CIDSTEM Labs