An innovative drug release system based on a modular technology has been developed at the University of Parma. The release modules, consisting in tablets or polymeric matrices, have been prepared to be assembled in a single structure, forming the drug release system. A range of configurations may be obtained based on the module assembling way. The module approach allows different drugs to be simultaneously administered at different rates and at targeted sites.
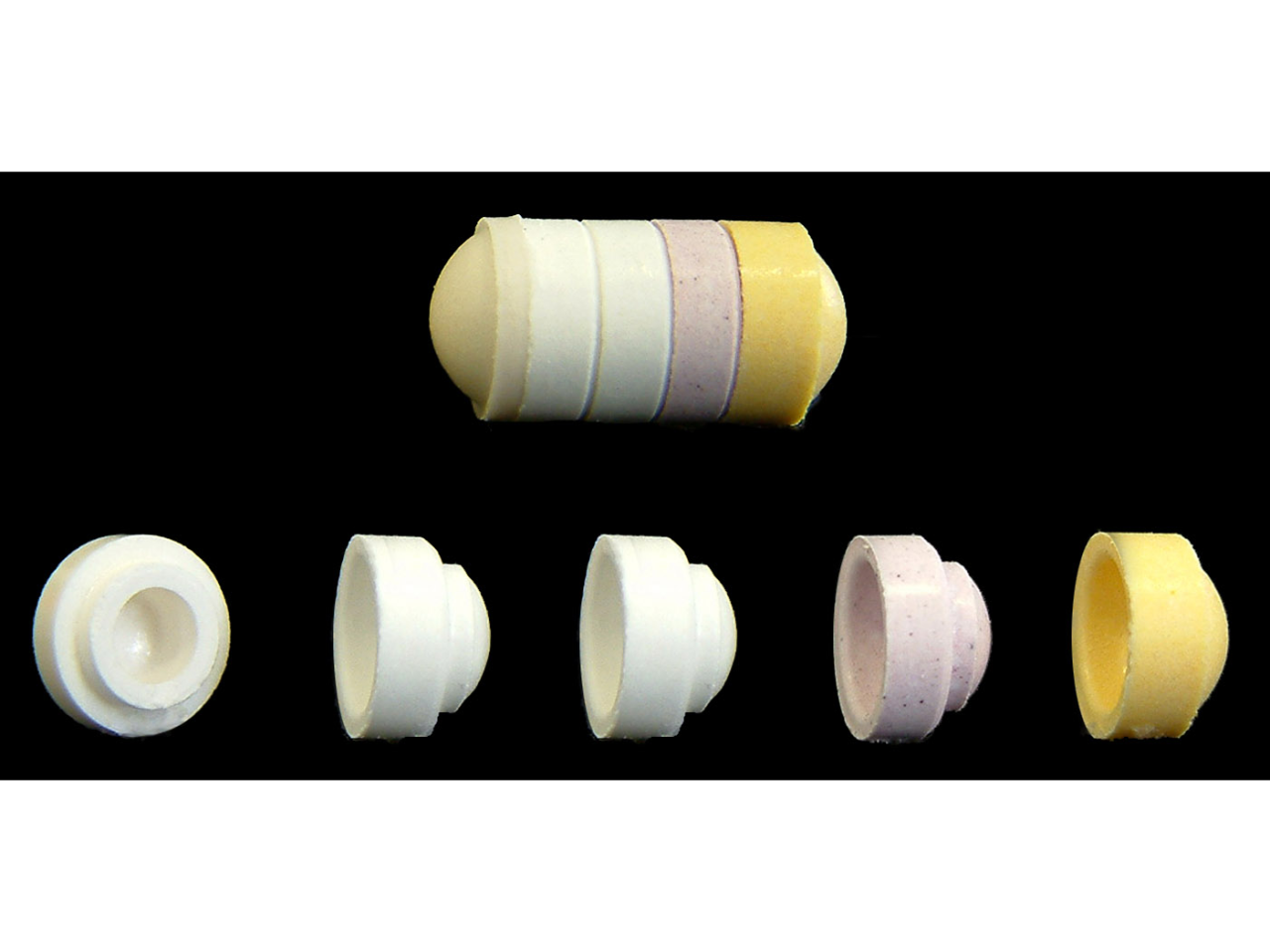 Five modules but a single assembly
Five modules but a single assembly
Different medications may be administered in a single pharmaceutical form at different rates in different modules. The most important innovation achieved consists in the gastric-retentive behaviour based on the floatation of two assembled modules with concave face to concave face. In vivo studies have confirmed the gastric-retentive ability of the empty chamber configuration. The system has remained in the stomach for a time between 2.5 to 5.0 hrs, depending on the subject's diet and gender.
The Dome Matrix technology is of interest for pharmaceutics companies that market prolonged release solid dosage forms. The release platform targets companies dealing with the production of conventional and bio-technological drugs. The technology addresses the need for versatility in drug release kinetics by modulating the administered dose and associating different drugs in a single system. Since the technology provides a time-space controlled release, it might also be used in agricultural, industrial and consumer applications.
Innovation in the treatment of malaria in adult and children patients: application of modified release technologies
Resistance to antimalarial drugs developed by the Plasmodium Falciparum micro-organism is the leading cause of treatment failure. The combination of two drugs such arthemisinin or its derivatives and other clindamycin, are a new tool with which to attempt combating resistant strains. A medicinal preparation able to control the release of the two drugs, simplifying administration to the patient, would provide a more effective antimalarial therapy. In fact, the complex posology of the therapy (many pills to be swallowed, each containing a single drug) often confuses and leads the patient to taking them incorrectly or occasionally. Constructing a multi-kinetic release system in the form of single dosage of an artemisinin and clindamycin combination, is a way to tackle this problem. The individual modules containing the two drugs will be constructed so that after combining they provide control over blood levels of the active substances, in order to reduce as much as possible the frequency of daily administrations and encourage the patient's adherence to the posology scheme. The product exists in prototype state. The pharmacokinetics study has not been carried out yet due to cost reasons. The pharmacokinetics study could be resumed following an investment.
Universiti Sains Malaysia
The technology is protected by two patents belonging to the Parma University. UNIPR has granted exclusive licence to develop products that use the technology to the pharmaceutical company Lisapharma spa, Erba (CO) which manages commercial agreements and has entrusted the development studies to BioPharmanet- tec.
 DomeMatrix® assembled system for malaria treatment
DomeMatrix® assembled system for malaria treatment