Biopharmanet-tec developed an innovative delivery system for the nasal vaccination using dry powders containing at the same time an antigen and an adjuvant in form of a nanoemulsion.
Powders have been obtained by «layering» the antigen and the nanoadjuvant on a carrier.
This carrier has been selected among hydrophilic pharmaceutical excipients able to preserve the characteristics of the antigen and of the adjuvant and to favor their dispersion upon contact with the surface of the upper airways
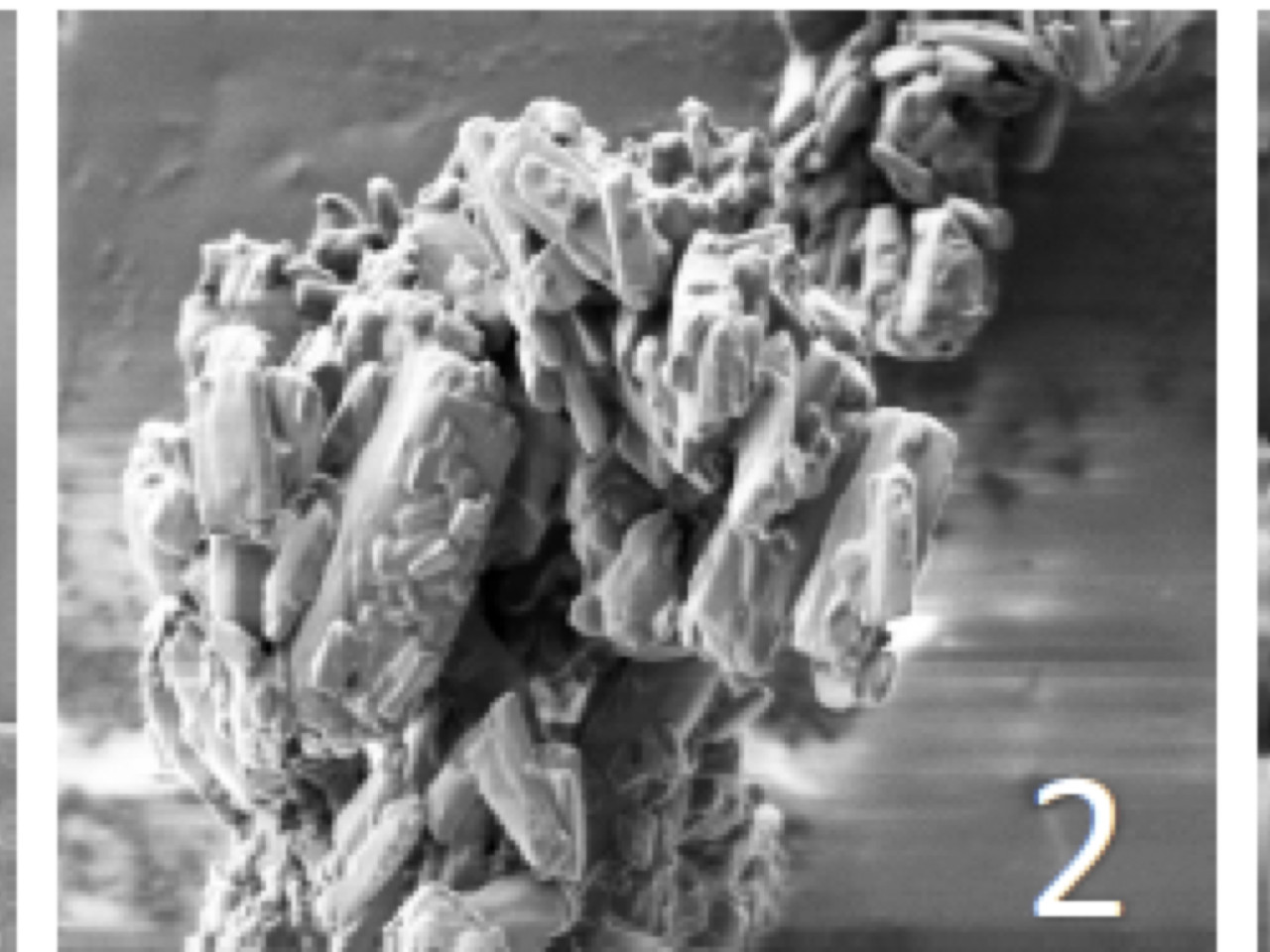 SEM images of particles obtained by deposition on mannitol of Mycoplasma a and nanoemulsion. Magnification x1000 (1), x5000 , x10000X (2) and 100000X (3).
SEM images of particles obtained by deposition on mannitol of Mycoplasma a and nanoemulsion. Magnification x1000 (1), x5000 , x10000X (2) and 100000X (3).
The product has to be sterile and refrigerated to maintain its properties. This require a strict control over the cold chain logistics and administration by qualified healthcare personnel. The use of a mucosal vaccination strategy, as in the case of nasal delivery, offer a safe and convenient alternative to patients avoiding parenteral injection. Use of a powder as dosage form limits stability issues. Moreover, the simultaneous administration of antigen and nanoemulson loaded on the carrier surface along with a mucoadhesive polymer such as chitosan elicit an adeguate immune response.
This technology can be applied in the prevention of human and veterinary infectious diseases. The technology platform can be employed for the delivery of small molecules or biotech drugs. The control over the final composite material is obtained through the selection of the excipients and of the carrier which aqueous solubility affects the interaction with mucosal surfaces
 Nasal powder insufflator (Miat, Italia) used in the animal studies evaluating the dry powder vaccination strategy
Nasal powder insufflator (Miat, Italia) used in the animal studies evaluating the dry powder vaccination strategy
Innovation in the prevention of lung infections in the veterinary field: the case of Mycoplasam Hyopenumoniae in piglets
M. Hyopneumoniae is the pathogen responsible for the swine enzootic pneumonia. This is an endemic and highly infectious disease affecting piglets in the first weeks impacting growth, morbidity, veterinary costs and overall the productivity of the animal farm. Since antibiotic treatment does not guarantee the infection eradication, vaccination appears the best approach. However, marketed vaccines are to able to prevent the airways colonization by Mycoplasma. The use of a nasal vaccine based on inactivated M. Hyopneumoniae appears as a promising strategy to overcome the limitations of vaccines currently in use.
The dry powder vaccine has been produced by layering on pharmaceutical excipients such as mannitol, lactose or calcium carbonate the antigen along with an adjuvant. This adjuvant is a nanoemulsion containing sunflower oil and chitosan, a polysaccharide known for its mucoadhesion properties. In animal studies conducted so far the powder is well tolerated and elicits an immune response comparable to marketed vaccinesa una risposta immunitaria comparabile a prodotti già in commercio.
Department of Veterinary Science, University of Parma
The technology is protected by a patent. Talks with potential partners pharmaceutical and veterinary companies are ongoing. Supplementary studies able to demonstrate the efficacy of the vaccine and for the scale–up of the manufacturing of «dry» vaccines will be the object of the industrial collaboration aiming at the development of the product.
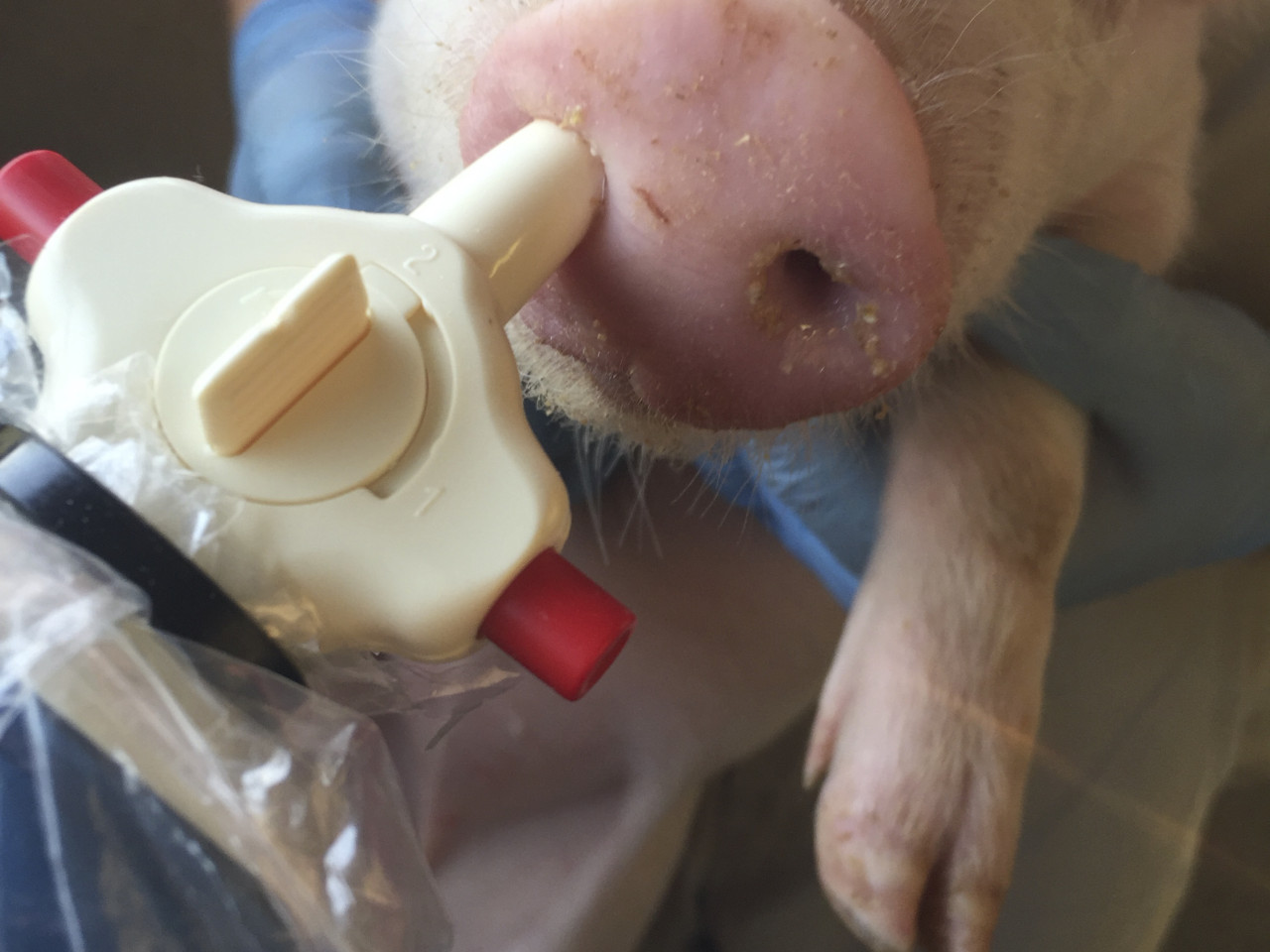 Nasal administration in piglets of the dry powder loaded with the antigen and the nanoemulsion adjuvant
Nasal administration in piglets of the dry powder loaded with the antigen and the nanoemulsion adjuvant