EDA is a device assisting in the correct self- or assisted-administration of eye drops, solving the very common problem of low adherence to eye drop treatment and incorrect techniques of eye drop administration.
Even though EDA projecting required complex morphologic analyses, the device is simple to use and to produce, and is compatible with all eye drop bottles.
 Eye Drop Aid
Eye Drop Aid
Eye diseases leading to hypovision and blindness (0.5-0.8% and up to 20% after the age of 70) are the object of prevention strategies often including the use of topic treatments.
According to the Italian Ministero della Salute (2018), chronic eye diseases are the main cause of blindness (84%) and the increasing incidence of degenerative diseases due to population aging leads to predict a 300% increase in people with vision problems in the next decades. Topic eye drop treatment is used for both acute and chronic conditions (glaucoma, allergies, dry eye, neurotrophic cheratitis). Various products are used, such as antimicrobial, antiinflammatory, antiallergy, moisturizing, antidistrophic, midriatic molecules. According to the WHO, the adherence to the therapy of chronic for ocular diseases is only about 50%.
In addition to the adherence issue, the eye drop self-administration is not always correctly performed or even possible, due to motor incoordination, wrong positioning of the eye drop bottle, hand instability during the bottle compression, undue contact of the bottle with eye surface or with skin. Possible consequences are: failed self-administration, risk of traumatisms, undue contact and risk of contamination of the product, loss of product for repeated attempts.
EDA may be a solution to these problems.
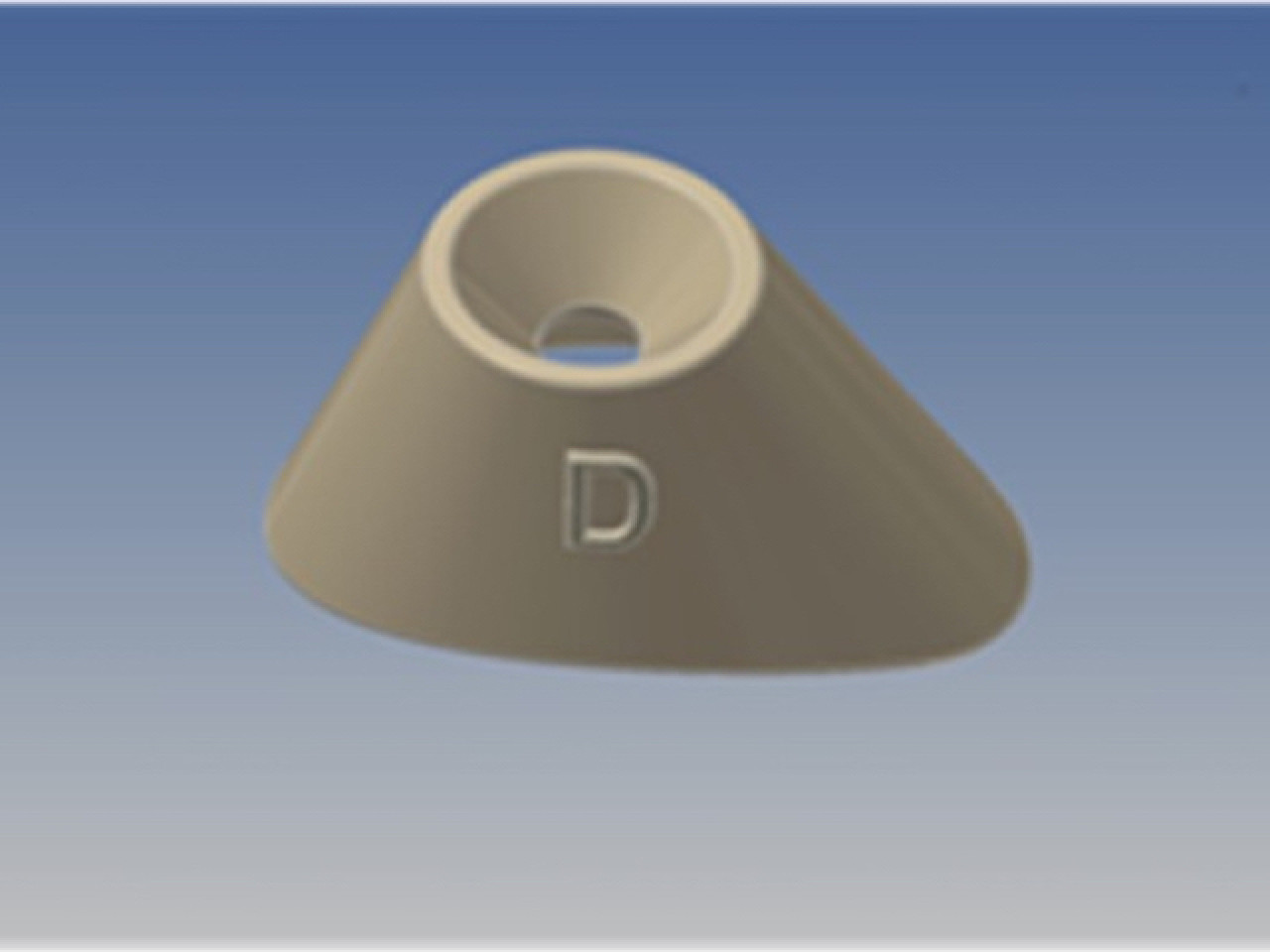
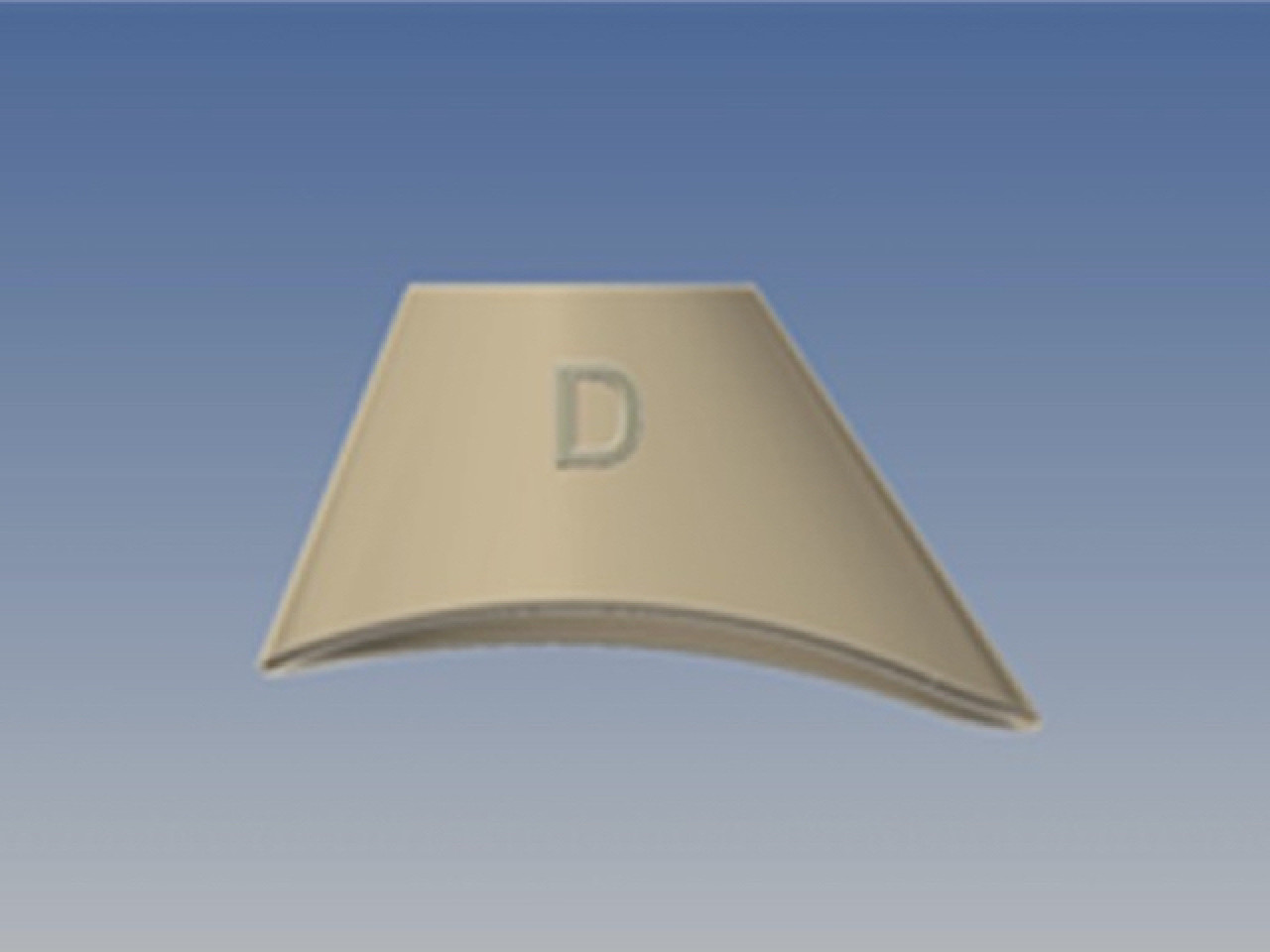
It is a completely original and innovative device, with the shape of a truncated cone, whose upper base is adapted to accommodate the dripper of a eye fluid dispenser and the lower base, not coplanar ovoid, is studied to adapt on the periocular area.
To date, no simple and universal device, compatible with all eye drop bottles, is commercially available for a simple and safe use in assisting or allowing eye drop self-administration.
EDA allowed the self-administration of eye drops in patients otherwise unable to perform such operation. It has also been appreciated by chronic treatment users, rendering eye drop self-administration faster, more correct and more precise, with less waist of product. Data, obtained in a small explorative sample, are currently being checked in a wider sample of subjects (MISE funding 2021).
 Eye Drop Aid
Eye Drop Aid
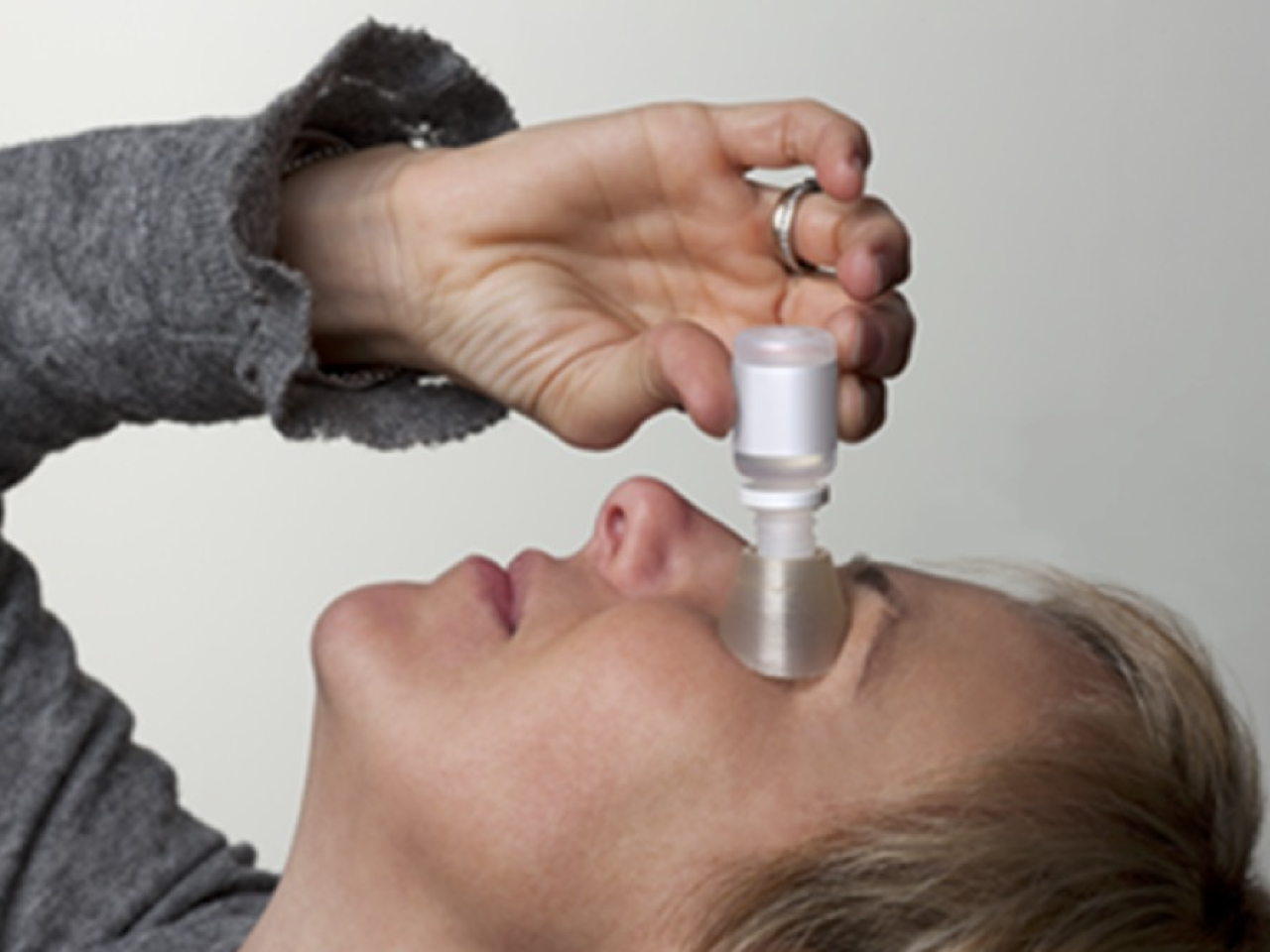
Self-administration and assisted administration of eye drops.
EDA:
-allows the quick and correct positioning of the eye drop bottle even in persons with motor incoordination (e.g. aged people)
-provides the right distance between the dripper and the eye surface, preventing possible traumatism and eye drop contamination
-eye drop remains sterile, as there is no contact between the product and EDA: the bottle dripper goes through the hole and the drop falls directly in the conjuntival sac
-avoids losses of eye drops
-gives a eye protection feeling during a bothersome procedure
The function of EDA is the result of a precise and complex design, based on eye and orbit anatomy. EDA may be produced in stereospecific form, right and left, or in universal form. The material is easy to shape, biologically unreactive, anti-bacterial, non toxic, pleasant to the touch, matt but semi-transparent; it does not produce sharp edges anti t is recyclable. EDA ha slow production costs and may be sold alone or within kits with eye drops or other devices for ocular treatment. Industrial production may be through injection molding or thermoforming, in polypropylene, or stereolithography, with materials in VI USP class.
No partner involved
EDA is protected by both an Italian ( IT201600102531A1 Dispositivo per somministrazione oculare di fluidi) and European patent (EP3308757A1 DEVICE FOR OCULAR ADMINISTRATION OF FLUIDS), the latter granted in October 2021.
 How to use Eye Drop Aid
How to use Eye Drop Aid