Holoclar® is a product of tissue engineering for advanced therapies consisting of an ex vivo reconstructed autologous human corneal epithelium containing stem cells, indicated for the reconstruction of the corneal surface in patients with limbal stem cell deficiency due to ocular burns. The active substance of Holoclar® is represented by the epithelial stem cells contained therein, that engraft and permanently regenerate a functional corneal epithelium allowing recovery of visual acuity. Holoclar® is prepared starting from a small sample of cells, obtained through the execution of a 1-2 mm2 biopsy of the undamaged limbus (the area of the cornea where the corneal stem cells are contained
 A cultured sheet of corneal epithelium (Holoclar®)
A cultured sheet of corneal epithelium (Holoclar®)
Holoclar®, who has obtained conditional marketing authorization by the European Commission in February 2015, is the first stem cell based medicinal product registered in the Western world. This success is entirely "made in Italy” and is due to the valuable combination between the scientific capabilities of world-renowned researchers, like Graziella Pellegrini and Michele De Luca of the University of Modena and Reggio Emilia, and the industrial capabilities and commitment to innovation of Chiesi Farmaceutici.
In a healthy eye the surface of the cornea – the transparent tissue located in front of the iris (the colored part of the eye) – constantly renews and repairs itself with new cells, produced by limbal stem cells, which are in the peripheral area of the cornea, called limbus. The ocular burns can damage these cells, preventing the proper regeneration of a healthy and transparent cornea. Holoclar® is indicated in all cases of moderate to severe limbal stem cell deficiency caused by chemical or thermal ocular burns. Such damage can affect one or both eyes, and can be superficial or deep.
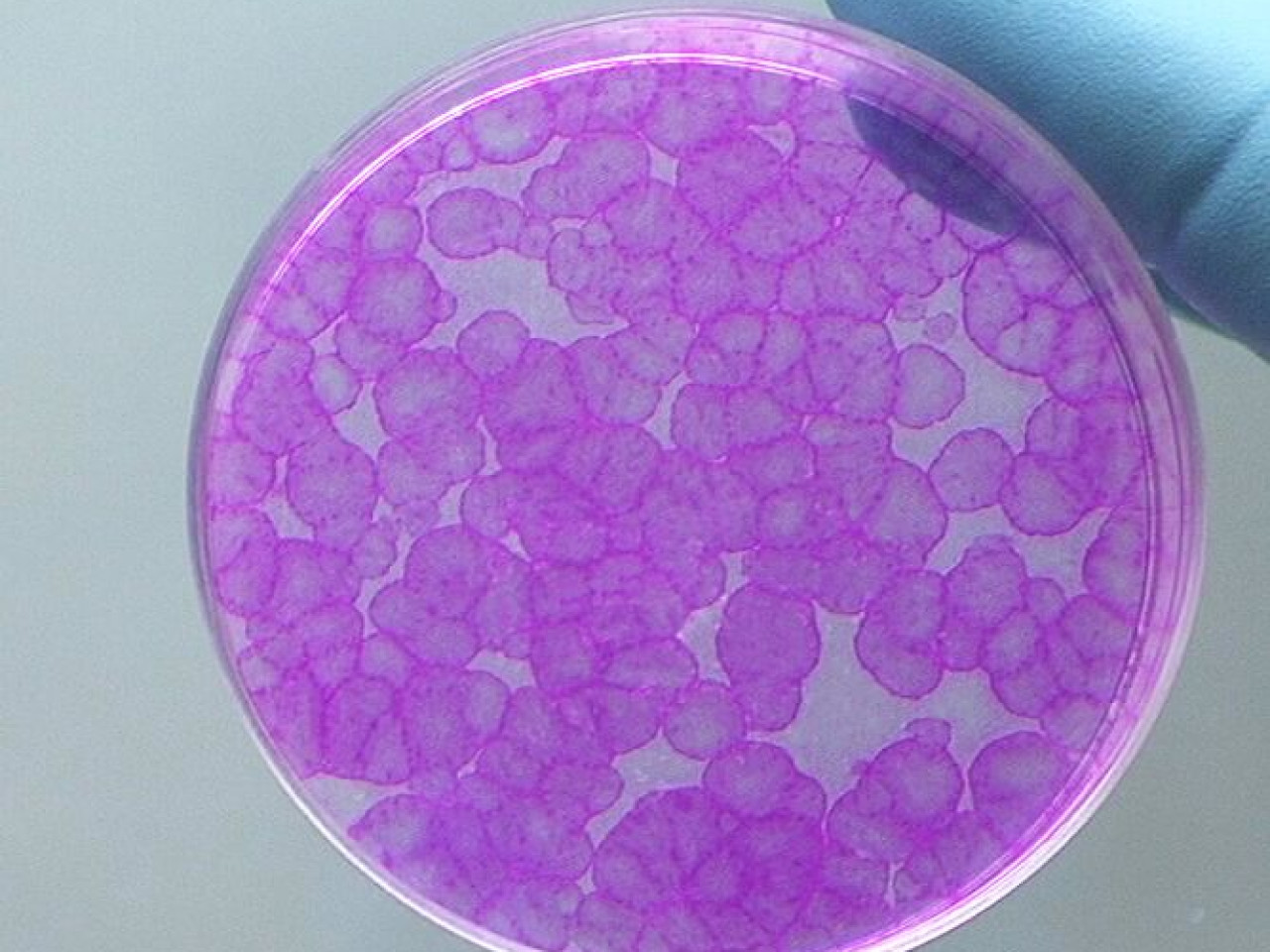 Colonies derived from a single epithelial stem cell
Colonies derived from a single epithelial stem cell
Clinical application in ophthalmology
The transplantation can occur even years after the accident, without being less effective. In general, prior to the intervention, the conditions of the eye must be brought back to normal: often burns affect not only the cornea, but involve the eye in its entirety. The environment in which the stem cells contained in Holoclar® are implanted should be as similar as possible to the healthy one: for example, the eyelids must be reconstructed, if damaged by the accident; lacrimation must be restored, inflammation cured. Only at this point the transplant can take place under ideal conditions, leading – in the case of superficial lesions – to a restoration of vision in approximately 80% of the cases treated. If the lesion is deep and also involves the part beneath the corneal epithelium, the grafting of Holoclar® is critical in order to be able to proceed, on average after 1 year, with the traditional corneal transplant. In fact, if the limbus is completely compromised, the corneal transplant is doomed to fail, because the implanted cornea is not covered by the corneal epithelium, as normally would happen, but by the cells of the conjunctiva, which make the cornea opaque. The advent of Holoclar® therefore maximizes the efficacy of the operation, by addressing another unmet need of these patients.
Holostem Terapie Avanzate s.r.l. (manufacturer) Chiesi Farmaceutici S.r.l. (Marketing Authorization holder)
De Luca M. and Pellegrini G., In vitro reconstituted sheets of human corneal epithelium and method of producing the same, EP Patent 1451302, May 28, 2008 De Luca M. and Pellegrini G., Reconstructed laminae of human epithelium corneae and method of producing the same, US Patent 6,610,538, Aug. 26, 2003
 Corridor in GMP area
Corridor in GMP area