Inhalable powder combining a local anaesthetic with a hydrophilic biocompatible polymer able to induce prolonged cough suppression
The powder is composed of microparticles suitable for the deposition in the upper airways where the cough receptors are localized and able to modulate the release of the local anaesthetic drug.
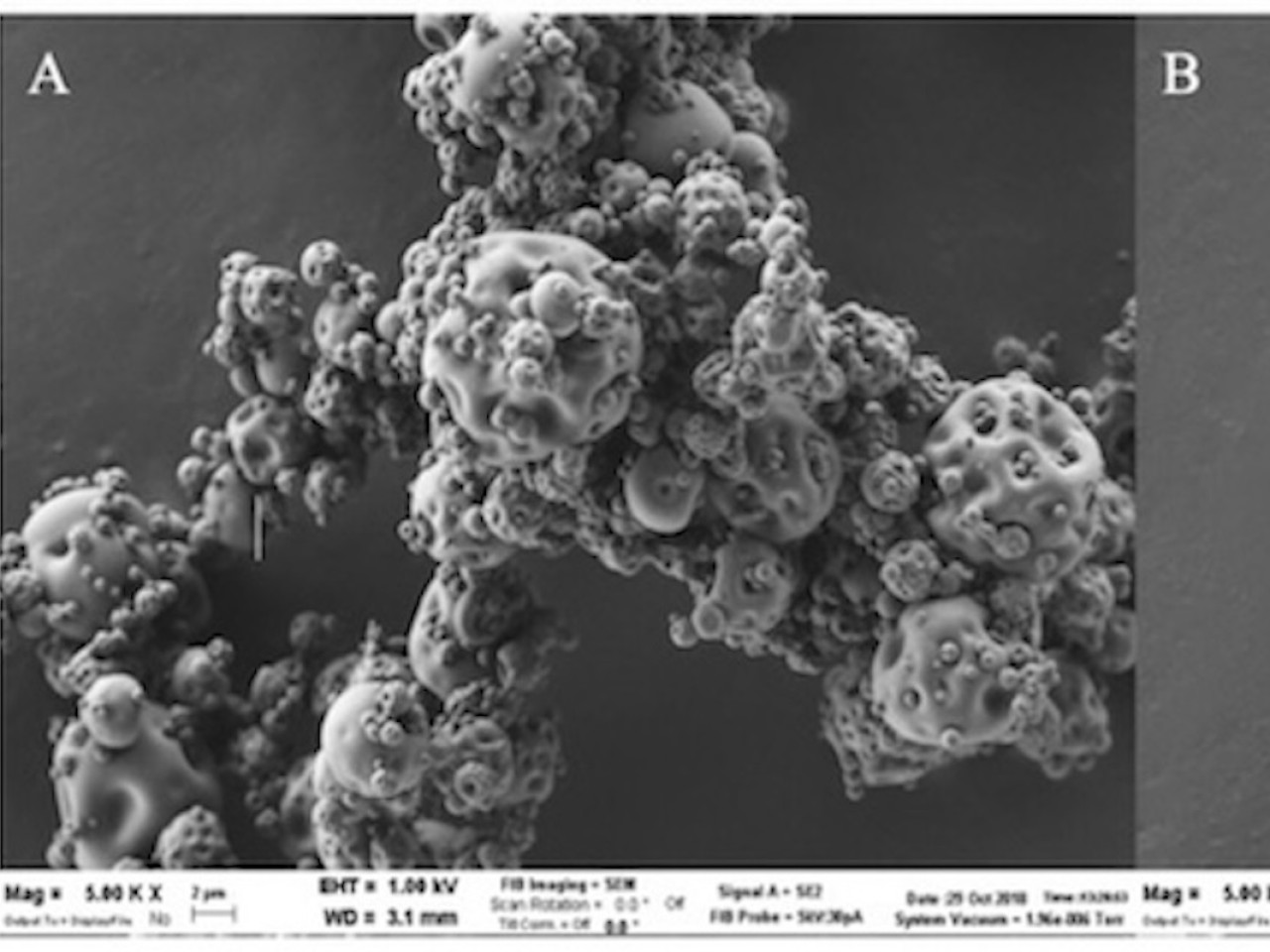 Scanning Electron Microscopy image of lidocaine-hyaluronic acid spray dried powder
Scanning Electron Microscopy image of lidocaine-hyaluronic acid spray dried powder
Cough is a common respiratory disorder for which many individuals seek medical advice. Chronic cough, that last longer than eight weeks, can be extremely harmful to the patient's life and it causes many clinical challenges. A proportion of patients with chronic cough have a persistent cough called as idiopathic chronic cough, that remains unexplained, and it occurs when the cough receptors are overly sensitive to stimuli produced by non-specific irritants.
Local anaesthetics (LANEs) could act as peripheral antitussives drugs that block neuronal sodium channels, reducing excitability of the nerves responsible for the cough reflex. The off label-use of LANEs in chronic cough has been investigated with a retrospective study demonstrating promising results for self-administration of nebulized lidocaine in adults.
The purpose of this research was to design and produce an inhalation powder, based on lidocaine, for the treatment of chronic cough. Lidocaine was spray dried with hyaluronic acid (HA) to obtain inhalable powders. Spray dried powders composed by HA at LMW with a lidocaine content lower than 20% afforded suitable dissolution profile and higher physical stability. The use of a polymer such as HA led to optimal entrapment of the drug allowing the production of amorphous particles and to modulate drug release.
The current treatment of chronic cough involves the use of centrally acting neuromodulator by oral route, such as amitriptyline, gabapentin, pregabalin and tramadol. At present, no inhalation product based on local anestetics is authorized for this indication.
The powder for inhalation developed is composed of lidocaine and hyaluronic acid with particles in a size range (6-12 µm) suitable for the deposition in the lung area where the cough receptors are localized. The presence a specific concentration of hyaluronic acid in the formulation allows to obtain amorphous microparticle which structure was maintained over time, and to achieve bioadhesion and a prolonged release of lidocaine and, as a result, efficient cough suppression.
The present invention concerns a combination of a local anaesthetic drug with a hydrophilic biocompatible polymer to be administered as a dry powder formulation for inhalation through a dry powder inhaler for the treatment of chronic cough and the prevention of cough induced by pharmacological treatments, as well as for the treatment of acute cough, e.g. in the course of viral infections of the airways or in post-infectious cough.
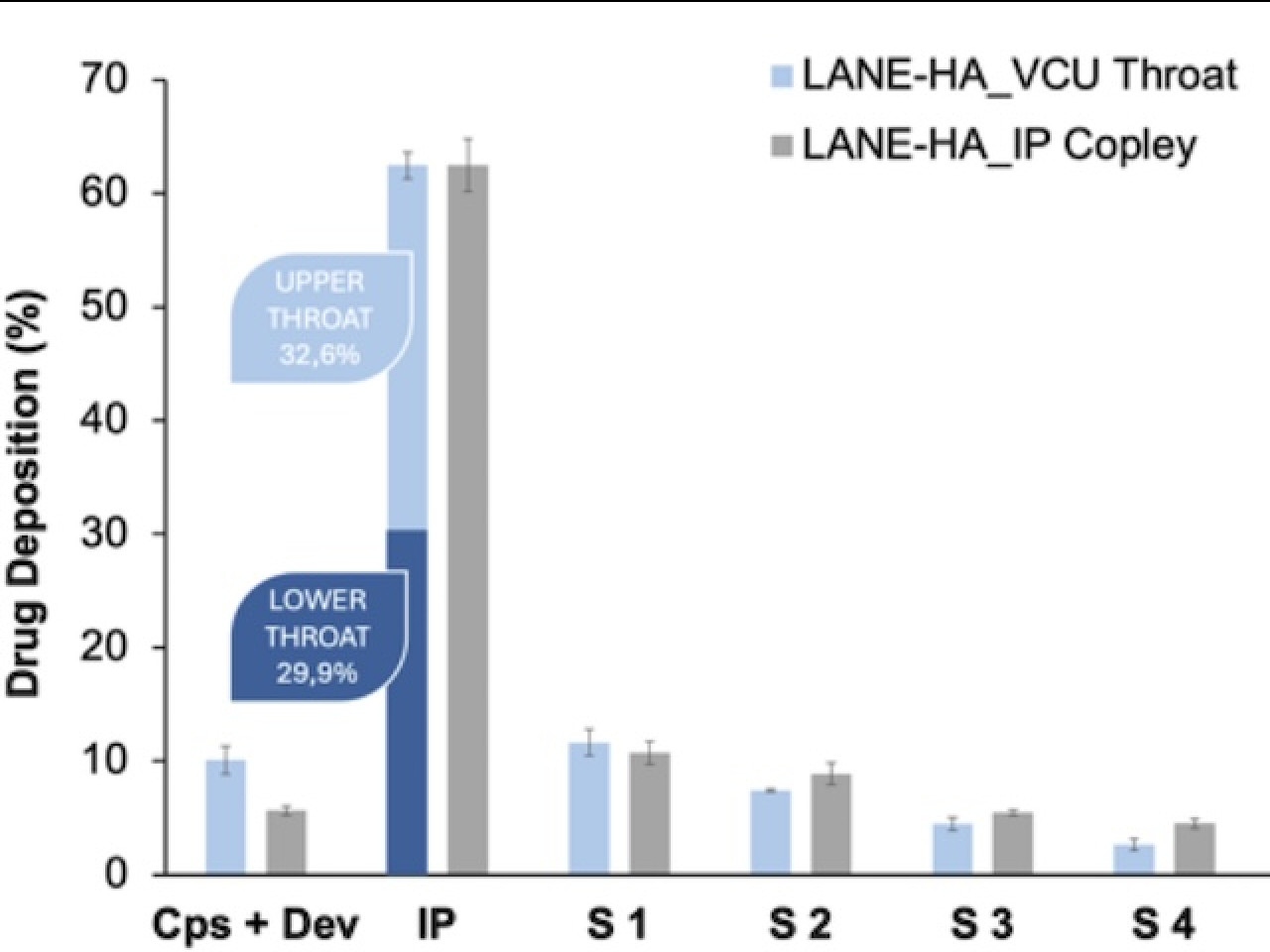 Next Generation Impactor deposition of lidocaine-hyaluronic acid spray dried powder using the Standard Induction Port (grey) and the VCU Throat model (blue).
Next Generation Impactor deposition of lidocaine-hyaluronic acid spray dried powder using the Standard Induction Port (grey) and the VCU Throat model (blue).
Production by spray dyring of inhalable powders containing a local anesthetic in combination with a hydrophilic biocompatible polymer and study of their efficacy for cough suppression in healthy volunteers
Spray dried powders composed by low molecular weight (LMW) hyaluronic acid (HA) with a lidocaine content lower than 20% provided the desired dissolution profile and physical stability. Aerodynamic performance of powders produced was tested using Next Generation Impactor employing a USP standard Induction Port or a realistic mouth-throat model. Similar patterns of deposition were evidenced with both geometries, with high deposition at the throat level (about 60%) and minimal deposition in deeper stages, indicating a potential targeting of upper airways where cough receptors are localized and a low systemic absorption responsible for side effects.
The release profile of lidocaine raw material and of lidocaine from the formulation was conducted using RespiCell™ an apparatus designed by University of Parma for in vitro dissolution test. The dissolution of the spray-dried powder was significantly slower (120 vs. 15 minutes) than lidocaine raw material.
Finally, a study was conducted on healthy volunteers to whom cough was induced by inhaling distilled water (Careggi Hospital, Florence, Italy). The “urge to cough” (UTC) was assessed by means of a visual analogue scale. The study showed that the inhalation of the powder significantly increased cough threshold and UTC in subjects tested with a median increase of 2.13-fold and an average duration of the effect of 50 ± 8 min.
Università di Firenze, Azienda Ospedaliero Universitaria Careggi
The inhalation powder containing local anesthetics is protected by an Italian patent (n°IT202100031637A1) registered in June 2023 and extended as an international application in June 2024.
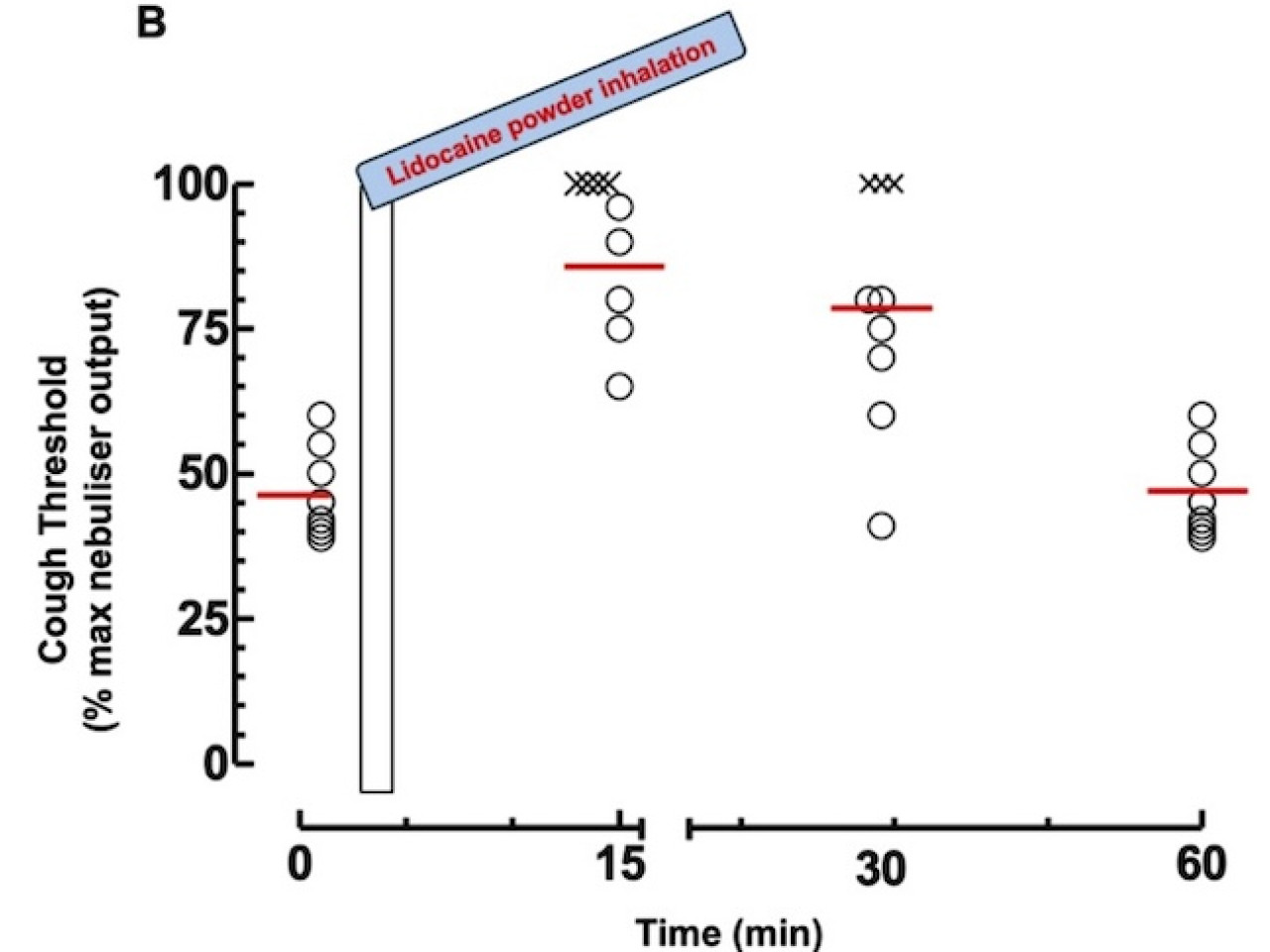 In vivo cough threshold output at different time points after lidocaine powder inhalation in healthy volunteers
In vivo cough threshold output at different time points after lidocaine powder inhalation in healthy volunteers