TECNO_EN-P, a system to create smart membranes
 Logo TECNO_EN
Logo TECNO_EN
The functionalized smart materials could favor the development of new MD with better therapeutical performance, that can be used for different application in the biomedical and clinical field. In fact, until today, the removal of cells and other biological components is carried out by non-selective technologies and this can cause the loss of different biological elements. Thanks to the set-up of smart materials, that act in a selective way, only the interested elements will be selected ensuring a safer treatment for the treated patient.
Thanks to the use of these kind of smart materials, a new generation of medical devices will be developed with a controllable specificity and higher selectivity/performance compared to the solutions currently on the market. This will allow the set-up of specific and personalised therapeutical approach.
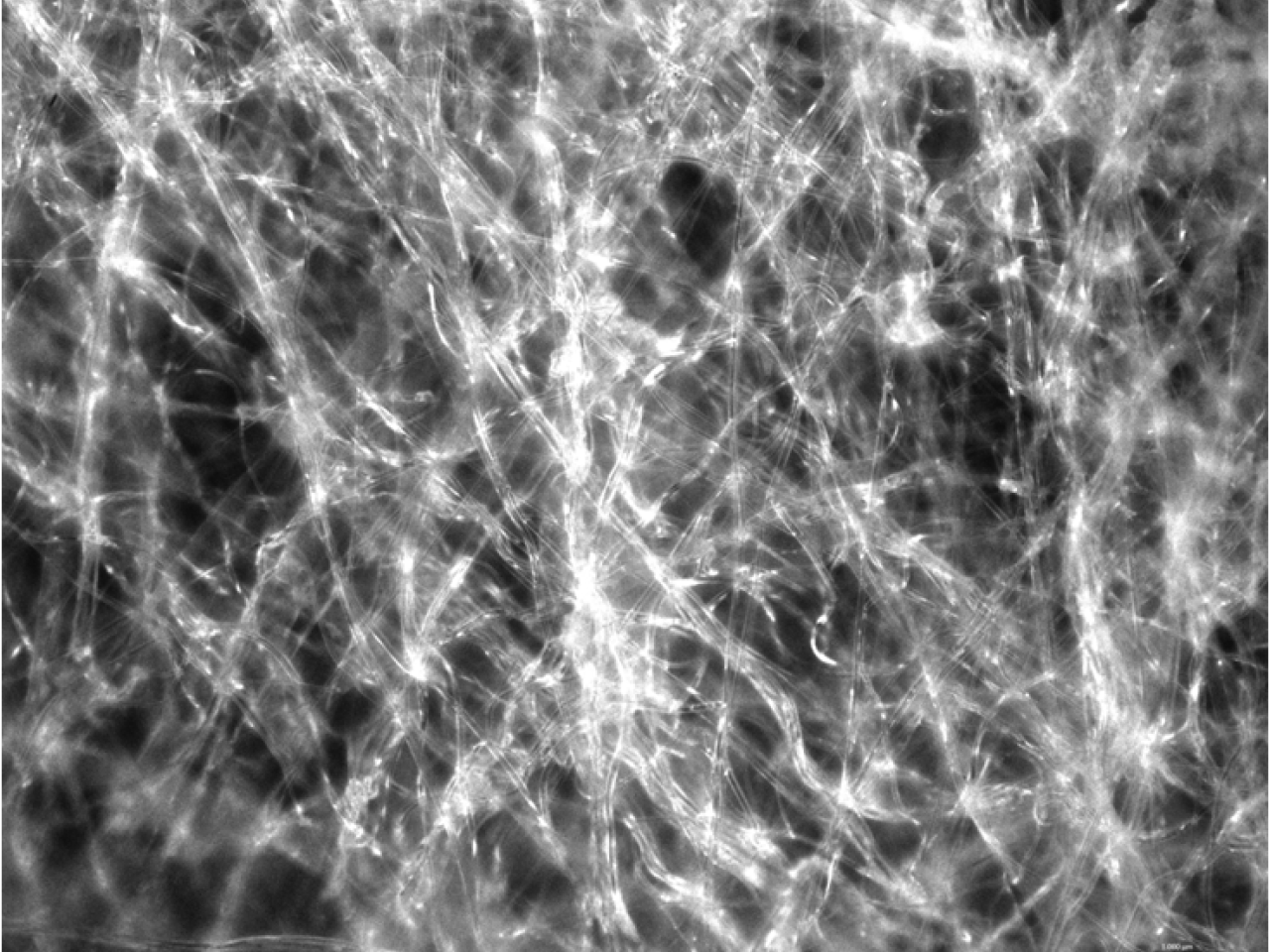 Imagine of biocompatible and filtering material
Imagine of biocompatible and filtering material
New technologies for innovative and smart membranes
TECNO_EN-P aims to develop new technologies to improve existing filtration systems. Until today, 4 different applications are identified: Specific selection of mesenchymal stem cells from adipose tissue (MSC); Selective removal of circulating tumour cells from intraoperative recovery blood; Selective removal of lipid suspensions from intraoperative blood; Removal of precipitate in therapeutic apheresis systems. The obtained solutions can enhance some therapeutic approaches (in certain pathologies where the removal of biological material / cells is the therapy) and the diagnostic methods through the analysis of the selected and isolated material. The evaluation of these smart membranes consisted of: - Removal performance, to evaluate if the performances are compatible with the specific areas of application; - Safety, through biocompatibility tests required by ISO 10993 and according to innovative approaches with tests developed at the TPM to obtain innovative devices applicable to the medical-health sector; - Production method, that has to be compatible with the existing one and considering different aspects such as sterilization, transport, storage and final cost of the product.
CIRI SdV CIRI MAM FONDAZIONE DEMOCENTER-SIPE
Four prototypes are made for different applications; patent application ongoing for one of them
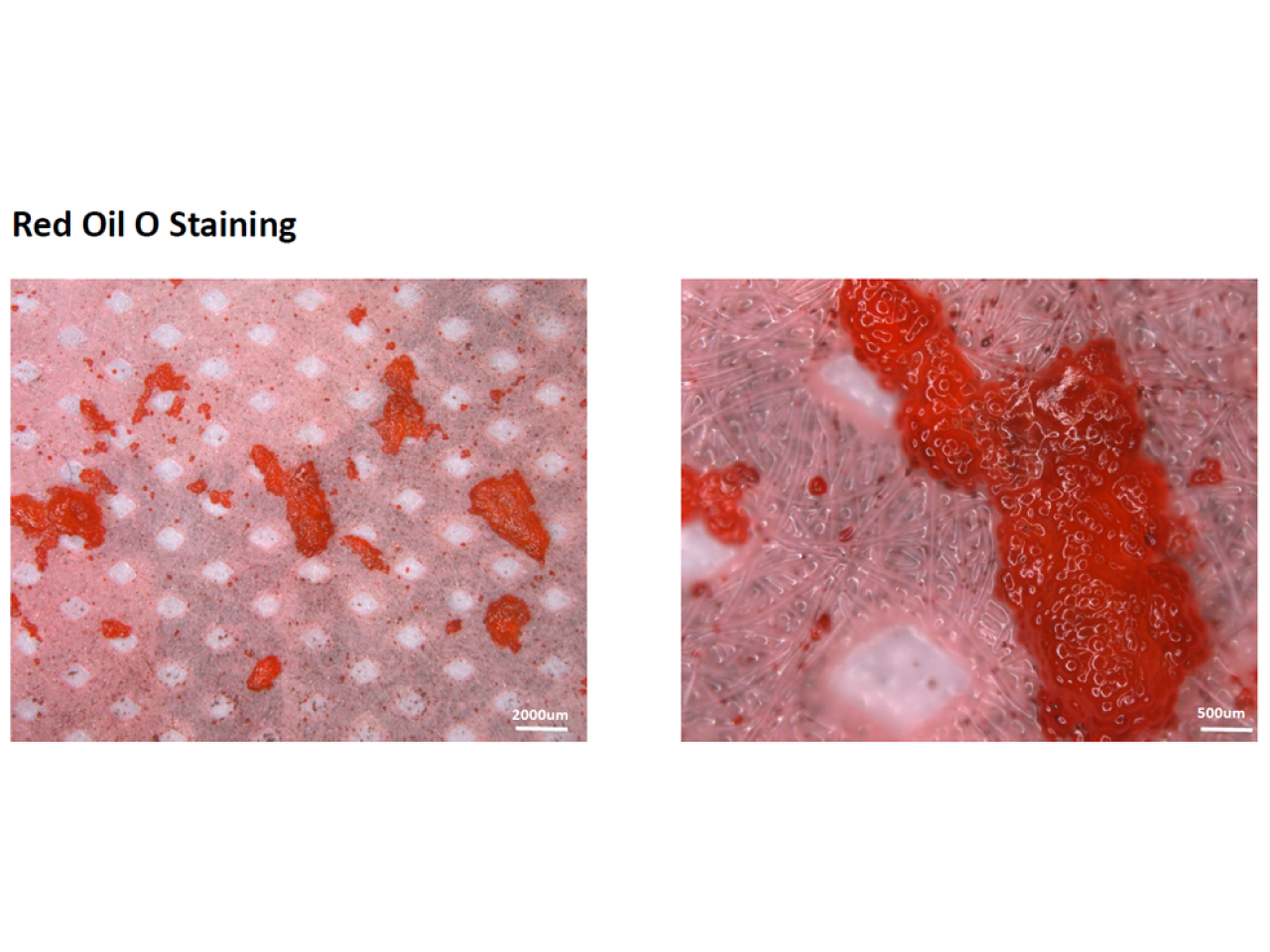 Functionalized membranes for lipids sequestration
Functionalized membranes for lipids sequestration