The Chamber for Environmental Control (CEC) is a technology designed to induce dry eye disease (DED) in mouse models, enabling highly controlled preclinical studies. This technology makes it possible to induce environmental stress conditions, such as low humidity and constant airflow, that replicate the natural risk factors of the disease without having to resort to invasive interventions, such as the removal of tear glands or the use of chemical agents. The flexibility of the CEC also enables the adaptation of environmental parameters, such as air quality and composition, thus turning it into an ideal tool for assessing the impact of air pollutants on the ocular surface. These features make it a versatile platform for the development and preclinical evaluation of new drugs and for studying interactions between the environment and ocular health.
 IRET Foundation, managing body of the Bologna-Ozzano “Rita Levi-Montalcini” Technopole
IRET Foundation, managing body of the Bologna-Ozzano “Rita Levi-Montalcini” Technopole
The Chamber for Environmental Control (CEC) is an experimental chamber designed to induce dry eye conditions in animal models. The CEC maintains strictly regulated environmental parameters and a constant air flow (8 L/min) to simulate desiccant stress. The workflow involves several hours per day of exposure in the CEC alternating with hours in their own cage for all animals involved. This reduces stress for the animals but ensures that corneal damage develops over a period of up to 21 consecutive days of exposure. The damage is quantified by a fluorescence test that visualizes the corneal surface. This is photographed and analyzed by quantifying the extent of damage with the NEI (National Eye Institute) scale. This modality is widely used in the assessment of corneal damage and dry eye severity in experimental models and in clinic. This workflow, which combines the use of the CEC with the use of the NEI scale, ensures a rigorous and standardized analysis, improving accuracy in measuring the impact of controlled exposures and tested treatments. Therefore, this tool is particularly suitable in the study of innovative efficacy therapies to reduce or prevent the appearance, extension and progression of corneal damage, capable of providing objective and reproducible data.
This instrument can handle groups of up to 15 animals simultaneously, with precise control of humidity, temperature and airflow to replicate eye stress conditions. No further invasive procedures on animals are required. After a minimum of 14-21 days of induction (depending on the targeted severity), the damage is quantifiable and the system can be used to evaluate the efficacy of ophthalmic drugs, or the effects of cosmetics or air pollutants. The model has clinical relevance as it reproduces the characteristics of human dry eye syndrome in animal models, validating treatments in a realistic way.
Possible applications include:
1) Preclinical studies of drugs for dry eye syndrome.
2) Investigation of the impact of air pollutants on ocular health.
3) Measurement of the effects of exposure to extreme climatic conditions.
 Chamber for Environmental Control for experimental models of dry eye
Chamber for Environmental Control for experimental models of dry eye
Comparative evaluation of the efficacy of different eye drops for the treatment of dry eye.
The CEC and its workflow were successfully applied, in collaboration with Thea Farma, to evaluate the efficacy of a new eye drops based on trehalose, hyaluronic acid and hydrocortisone. At the end of the damage induction period, it was possible to apply the inclusion criteria (damage > 30%) and to start comparative treatments. After 14 days, the results showed a 50% reduction in corneal damage (measured with fluorescein) in animals treated with eye drops (three times per day). A decrease in inflammatory markers (IL-1β and TNF-α) and an improvement in epithelial cell morphology were also observed. These data demonstrated the efficacy of the drug and the relevance of HCC as a preclinical screening tool, suggesting new comparative trials with different treatments.
Astolfi G, Lorenzini L, Gobbo F, Sarli G, Versura P. Comparison of Trehalose/Hyaluronic Acid (HA) vs. 0.001% Hydrocortisone/HA Eyedrops on Signs and Inflammatory Markers in a Desiccating Model of Dry Eye Disease (DED). J ClinMed. 2022 Mar 10;11(6):1518. doi: 10.3390/jcm11061518. PMID: 35329844; PMCID: PMC8948919.
Thea Farma is the Italian branch of Laboratoires Théa, an independent family-owned pharmaceutical company with over 30 years of experience in eye care.
University of Bologna: 1. CIRI SDV; 2. DIMEC (UO Ophthalmology - Prof. Versura); 3. DIMEVET (Pathological Anatomy Service – Prof. Sarli)
The validated technology is currently being used to test the efficacy of other medical devices designed for dry eye.
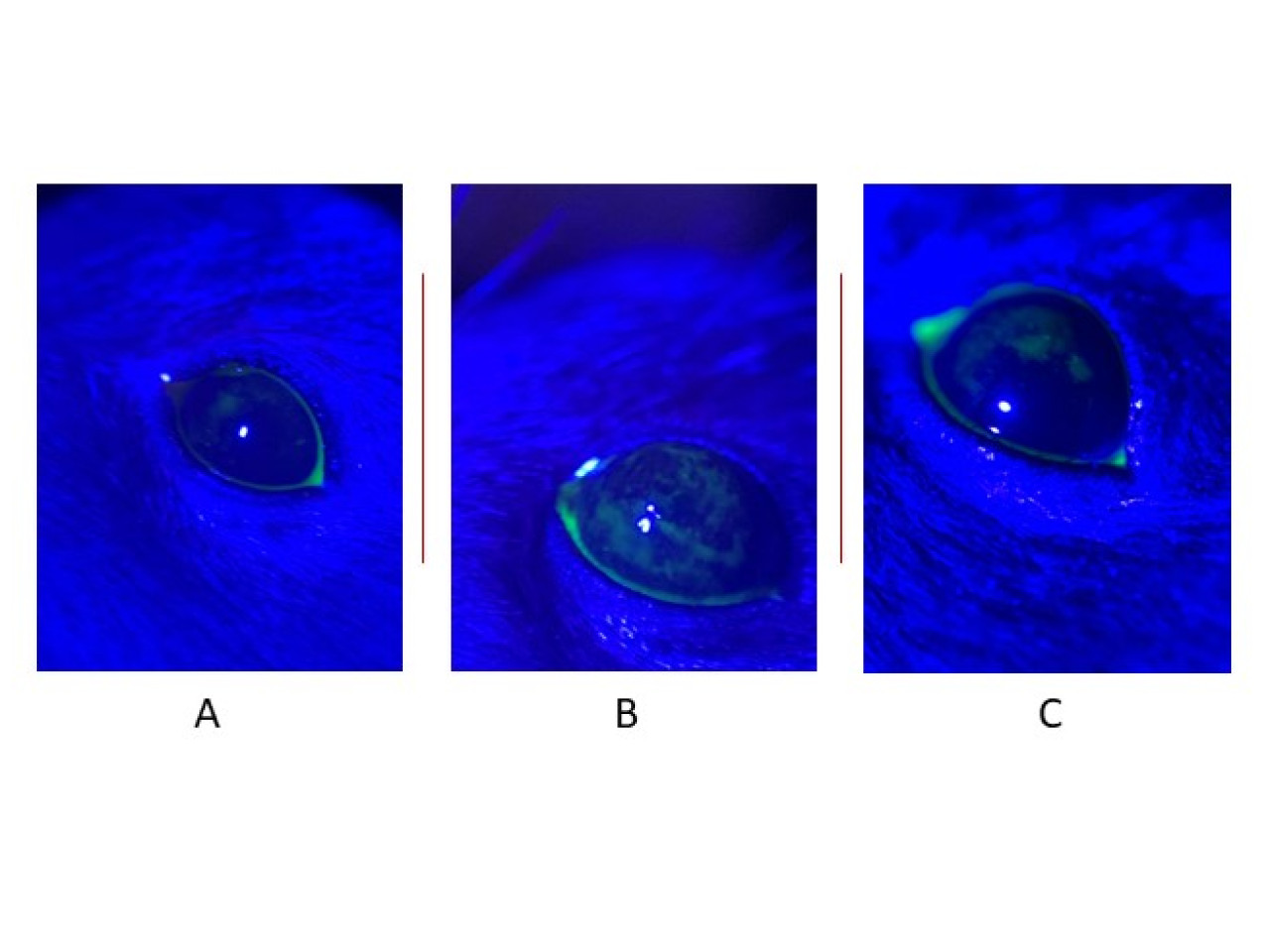 Integrity of the corneal epithelium with fluorescein, observed under slit lamp (cobalt blue filter): A. Baseline B. Post induction (21 days). C. Post drug treatment (21 days)
Integrity of the corneal epithelium with fluorescein, observed under slit lamp (cobalt blue filter): A. Baseline B. Post induction (21 days). C. Post drug treatment (21 days)