The electrochemical approach to water purification is based on the application of an electrical current, therefore the process is remotely controllable and neither personnel intervention nor the use of chemicals are required. The current disinfection method with chlorine dioxide and/or sodium hypochlorite may be replaced through the optimisation of electrochemical drinking water treatments. Electrochemical oxidation of the chlorides generally already present in the water allows, in fact, the external addition of oxidants to be avoided; the disinfecting result is achieved through the synergistic action of the in situ generated oxidizer and secondary effects such as electric field and sudden pH change.
 Technical instruments at the Terra&Acqua Tech Laboratory
Technical instruments at the Terra&Acqua Tech Laboratory
Synthesis of antimicrobial reactive species without using chemical additives, at the same time avoiding the formation of undesired substances. The process requires accurate optimisation of experimental conditions, and the development of electrode materials suitable for this specific use.
Water purification treatments in small aqueducts and/or individual facilities (hospitals, nursing homes)
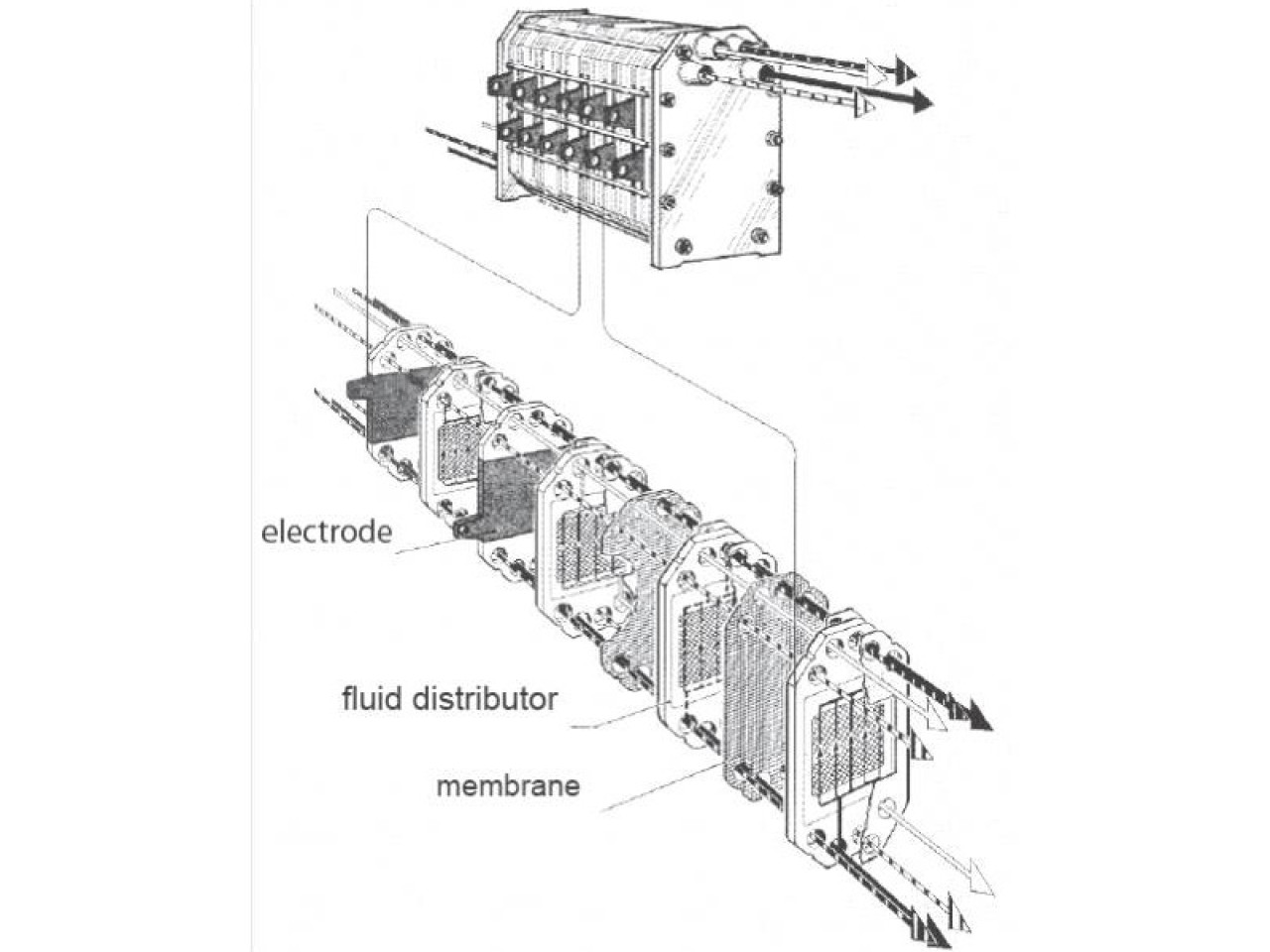 Operation diagram of the optimised electrochemical cell (from Elettrocell A/S, Denmark)
Operation diagram of the optimised electrochemical cell (from Elettrocell A/S, Denmark)
Ensure drinking water standards in the most peripheral areas of the Ferrara water mains
Chlorine is mostly added to the water mains of the Ferrara province in the main supply point of the network, where the drawn water is treated. At the secondary supply points further additions are made with chlorine dioxide (ClO2) or sodium hypochlorite (NaOCl). One of the main problems related to the added chlorine decay and transformation processes, is represented by the presence of chlorine by-products, both organic and inorganic. The reaction of chlorine with organic matter, naturally present in the water, may lead to the formation of products harmful to humans if present in high concentrations. With the aim of reducing these concentrations, without affecting the disinfection efficiency of water mains, an electrolytic process may be used for the production of hypochlorous acid, HOCl, obtainable by means of anodic oxidation half-reaction of the chloride anion. Test of the micro-pilot plant which consists of a modular reactor able to treat up to 15 litres of water per minute
Ferrara University (Electro-chemistry Lab. of the Chemical and Pharmaceutical Sciences Department, and "Water mains" Research Unit of the Engineering Department); HERA S.p.A.
Presentation at the 64th Annual Meeting of the International Society of Electrochemistry (Santiago de Queretaro, Mexico). The electrode devices may be subject to patent application.
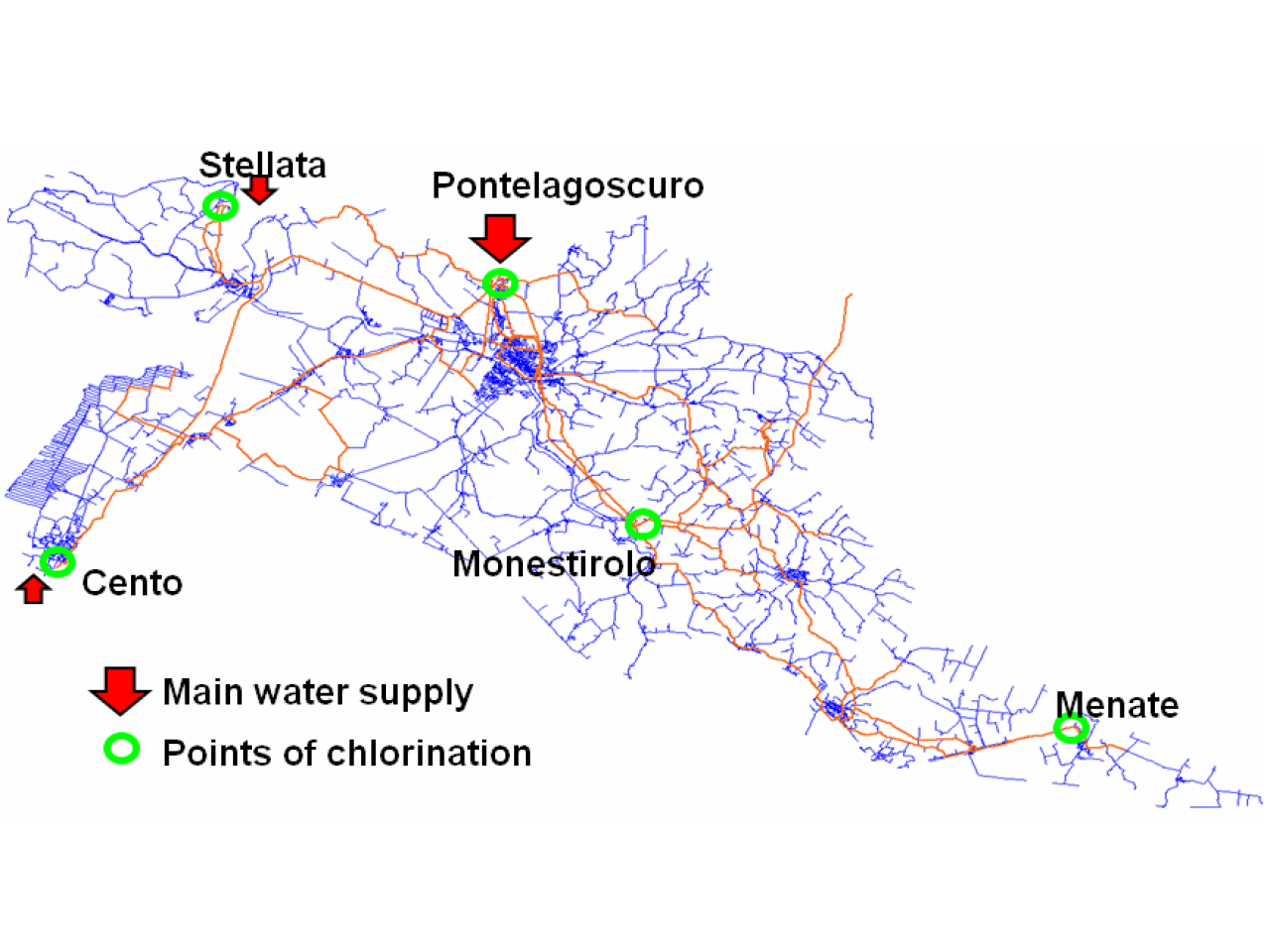 Water distribution network of the Ferrara area
Water distribution network of the Ferrara area