The service includes efficacy and safety certified studies according to the Good Laboratory Practice (GLP). It is optimized for the results validation and for the IND (Investigational New Drug) application. It includes the study design based on a primary and exploratory endpoints, power calculation for the sample determination and readouts aligned to the relative gold standards.
The service is applied to the development of anti-Alzheimer, neuroprotective and remyelinating drugs; enzymatic and genetic therapies for rare diseases; drugs and biomaterials for topical use for the repairment of hard-to-heal wounds, including the diabetic foot.
It is also applied to efficacy and safety studies for electro-medical devices (electromagnetic fields, laser, radiofrequencies) for systemic and loco-regional applications).
 The IRET Foundation is the Ozzano dell’Emilia branch of the Bologna Technopole named after Rita Levi Montalcini.
The IRET Foundation is the Ozzano dell’Emilia branch of the Bologna Technopole named after Rita Levi Montalcini.
The service is optimized to provide results derived from a robust statistical design based on power analysis to pharmaceutical and biomedical companies. The results are achieved in the minimal time, previewed by the experimentation, and presented as “proof of concept” containing original data sets and statistical analysis. This allows the inclusion in the test report for the IND.
The service is currently applied for lesions and diseases in the neurological field (neurodegenerative diseases, traumatic and vascular lesions of the nervous system, neuropathic and inflammatory pain), rheumatologic (arthritis), endocrine-metabolic diseases, epithelial lesions (pressure ulcers, diabetic foot, corneal lesions), rare diseases.
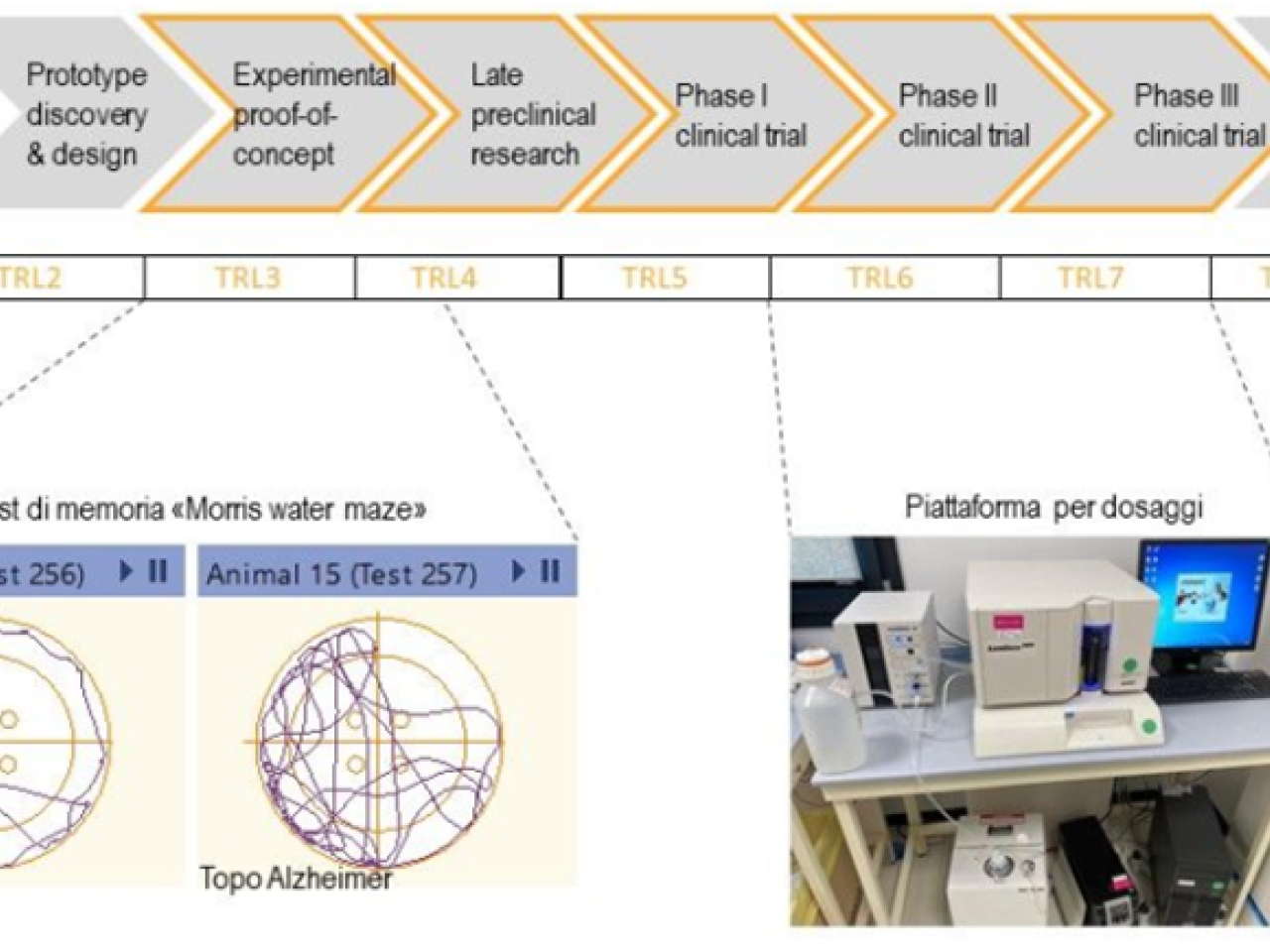 IRET Foundation offers the service for developing products from preclinical (TRL3) to clinical study (TRL7), using validated procedures according to the guidelines of the relative regulatory agencies.
IRET Foundation offers the service for developing products from preclinical (TRL3) to clinical study (TRL7), using validated procedures according to the guidelines of the relative regulatory agencies.
Development of an anti-Alzheimer molecule.
In 2006, Chiesi Farmaceutici contacted IRET Foundation for a preclinical study relative to a new gamma-secretase modulator (CHF7054) for the Alzheimer’s dementia therapy. They requested efficacy proofs in a preclinical model of the disease (transgenic mouse) regarding memory and disease biomarkers (AB40/AB42 and Tau/Ptau levels in blood and cerebrospinal fluid, amyloid plaques deposition in cerebral tissues, neuroinflammation) aimed for IND (Investigational New Drug). Between 2010 and 2014, the results have been validated through the publication on the major international journal in the field, allowing the launch of new phase I and II clinical studies (ClinicalTrials.gov Identifier:NCT01303744, NCT01203384, NCT01258452, NCT01602393) in which IRET Foundation was involved for the dosage of biomarkers using validated protocols (EMA and FDA).
In 2010 Chiesi obtained the approval from Emilia-Romagna Region (“Dai distretti produttivi ai distretti tecnologici”) for the project “Innovative technologies for the causal therapy of neurodegenerative diseases” for the development of the CHF5074 molecule. Three laboratories of the network were included in the study (CIRI-SDV University of Bologna; LTTA University of Ferrara; IRET Foundation, Ozzano Emilia). In 2014 Chiesi sold the molecule to an American start-up (transfer of license), build up for the phase III development of the molecule.
Chiesi Farmaceutici; CIRI-SDV University of Bologna; LTTA, University of Ferrara
The gained experience in developing the product derived services to other pharmaceutical (N=3), biomedical and electro-medical (N=3) companies. Moreover, the spin-off TransMed Research has been founded to manage the GLP certified activities according to AIFA, EMA and FDA disciplinary.
 Development, validation and valorization of the CHF5074 product, from TRL 3 to TRL 7.
Development, validation and valorization of the CHF5074 product, from TRL 3 to TRL 7.